(a) Calculate AH for the producti this reaction. (b) Calculate AH for the production of 9.00 g of AgCl. (c) Calculate AH when 9.25 × 10¯4 mol of AgCl dis- solves in water. 46 At one time, a common means of forming small quantities of oxygen gas in the laboratory was to heat KCIO3: 2 KCIO3(s) –→ 2 KC1(s) + 3 O2(8) AH = -89.4 kJ For this reaction, calculate AH for the formation of (a) 1.36 mol of O2 and (b) 10.4 g of KCl. (c) The decomposition of KC1O3 proceeds spontaneously when it is heated. Do you think that the reverse reaction, the formation of KC1O3 from KCl and O2, is likely to be feasible under ordinary conditions? Explain your answer. 7 Consider the combustion of liquid methanol, CH3OH(1): CH3OH(1) + ¿O2(g) → CO2(g) + 2 H2O(1) AH = -726.5 kJ %3D (a) What is the enthalpy change for the reverse reaction? (b) Balance the forward reaction with whole-number co- efficients. What is AH for the reaction represented by thi: equation? (c) Which is more likely to be thermodynami cally favored, the forward reaction or the reverse reaction (d) If the reaction were written to produce H,O(g) instea of H20(1), would you expect the magnitude of AH to ir crease, decrease, or stay the same? Explain. 8 Consider the decomposition of liquid benzene, C,H6(1), gaseous acetylene, C2H2(8): C,H6(1) → 3 C2H2(g) AH = +630 kJ %3D
(a) Calculate AH for the producti this reaction. (b) Calculate AH for the production of 9.00 g of AgCl. (c) Calculate AH when 9.25 × 10¯4 mol of AgCl dis- solves in water. 46 At one time, a common means of forming small quantities of oxygen gas in the laboratory was to heat KCIO3: 2 KCIO3(s) –→ 2 KC1(s) + 3 O2(8) AH = -89.4 kJ For this reaction, calculate AH for the formation of (a) 1.36 mol of O2 and (b) 10.4 g of KCl. (c) The decomposition of KC1O3 proceeds spontaneously when it is heated. Do you think that the reverse reaction, the formation of KC1O3 from KCl and O2, is likely to be feasible under ordinary conditions? Explain your answer. 7 Consider the combustion of liquid methanol, CH3OH(1): CH3OH(1) + ¿O2(g) → CO2(g) + 2 H2O(1) AH = -726.5 kJ %3D (a) What is the enthalpy change for the reverse reaction? (b) Balance the forward reaction with whole-number co- efficients. What is AH for the reaction represented by thi: equation? (c) Which is more likely to be thermodynami cally favored, the forward reaction or the reverse reaction (d) If the reaction were written to produce H,O(g) instea of H20(1), would you expect the magnitude of AH to ir crease, decrease, or stay the same? Explain. 8 Consider the decomposition of liquid benzene, C,H6(1), gaseous acetylene, C2H2(8): C,H6(1) → 3 C2H2(g) AH = +630 kJ %3D
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.103QE
Related questions
Question
5.46 (a) and (b)
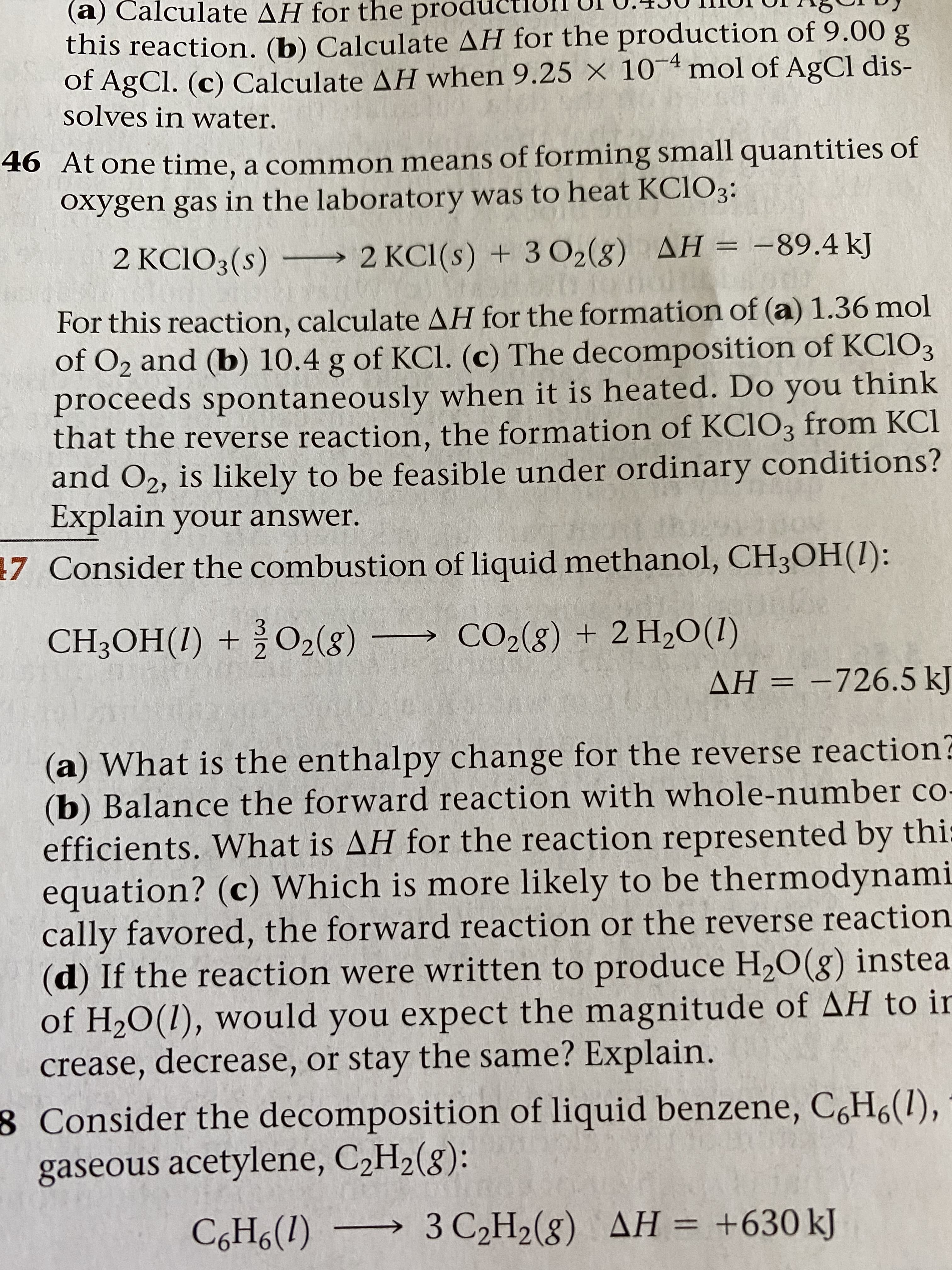
Transcribed Image Text:(a) Calculate AH for the producti
this reaction. (b) Calculate AH for the production of 9.00 g
of AgCl. (c) Calculate AH when 9.25 × 10¯4 mol of AgCl dis-
solves in water.
46 At one time, a common means of forming small quantities of
oxygen gas in the laboratory was to heat KCIO3:
2 KCIO3(s) –→ 2 KC1(s) + 3 O2(8) AH = -89.4 kJ
For this reaction, calculate AH for the formation of (a) 1.36 mol
of O2 and (b) 10.4 g of KCl. (c) The decomposition of KC1O3
proceeds spontaneously when it is heated. Do you think
that the reverse reaction, the formation of KC1O3 from KCl
and O2, is likely to be feasible under ordinary conditions?
Explain your answer.
7 Consider the combustion of liquid methanol, CH3OH(1):
CH3OH(1) + ¿O2(g)
→ CO2(g) + 2 H2O(1)
AH = -726.5 kJ
%3D
(a) What is the enthalpy change for the reverse reaction?
(b) Balance the forward reaction with whole-number co-
efficients. What is AH for the reaction represented by thi:
equation? (c) Which is more likely to be thermodynami
cally favored, the forward reaction or the reverse reaction
(d) If the reaction were written to produce H,O(g) instea
of H20(1), would you expect the magnitude of AH to ir
crease, decrease, or stay the same? Explain.
8 Consider the decomposition of liquid benzene, C,H6(1),
gaseous acetylene, C2H2(8):
C,H6(1) → 3 C2H2(g) AH = +630 kJ
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning