A certain catalyzed reaction is known to have an activation energy E 9.0 kJmol. Furthermore, the rate of this reaction is measured at 354. K and found to be 4.7 x 10 M/s. Use this information to answer the questions in the table below. Suppose the concentrations of all reactants is kept the same, but the temperature 5% from 354. K to 372. K. raised by choose one The rate will How will the rate of the reaction change? Suppose the concentrations of all reactants is kept the same, but the catalyst is removed, which has the effect of raising the activation energy by 5%, from 9.0 kJ/mol to 9.45 kJmol choose one The rate will choose one How will the rate of the reaction change? stay the same rise about 5% rise more than 5% rise less than 5% fall about 5% fall more than 5% fall less than 5%
A certain catalyzed reaction is known to have an activation energy E 9.0 kJmol. Furthermore, the rate of this reaction is measured at 354. K and found to be 4.7 x 10 M/s. Use this information to answer the questions in the table below. Suppose the concentrations of all reactants is kept the same, but the temperature 5% from 354. K to 372. K. raised by choose one The rate will How will the rate of the reaction change? Suppose the concentrations of all reactants is kept the same, but the catalyst is removed, which has the effect of raising the activation energy by 5%, from 9.0 kJ/mol to 9.45 kJmol choose one The rate will choose one How will the rate of the reaction change? stay the same rise about 5% rise more than 5% rise less than 5% fall about 5% fall more than 5% fall less than 5%
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 71QAP: For a certain reaction, Ea is 135 kJ and H=45 kJ. In the presence of a catalyst, the activation...
Related questions
Question
100%
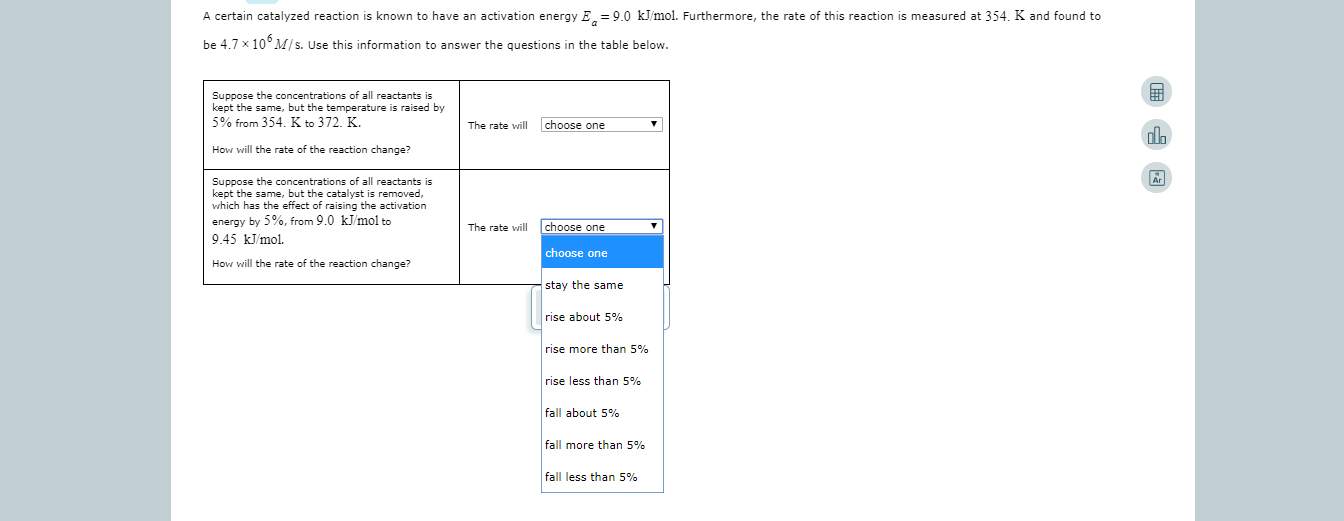
Transcribed Image Text:A certain catalyzed reaction is known to have an activation energy E 9.0 kJmol. Furthermore, the rate of this reaction is measured at 354. K and found to
be 4.7 x 10 M/s. Use this information to answer the questions in the table below.
Suppose the concentrations of all reactants is
kept the same, but the temperature
5% from 354. K to 372. K.
raised by
choose one
The rate will
How will the rate of the reaction change?
Suppose the concentrations of all reactants is
kept the same, but the catalyst is removed,
which has the effect of raising the activation
energy by 5%, from 9.0 kJ/mol to
9.45 kJmol
choose one
The rate will
choose one
How will the rate of the reaction change?
stay the same
rise about 5%
rise more than 5%
rise less than 5%
fall about 5%
fall more than 5%
fall less than 5%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning