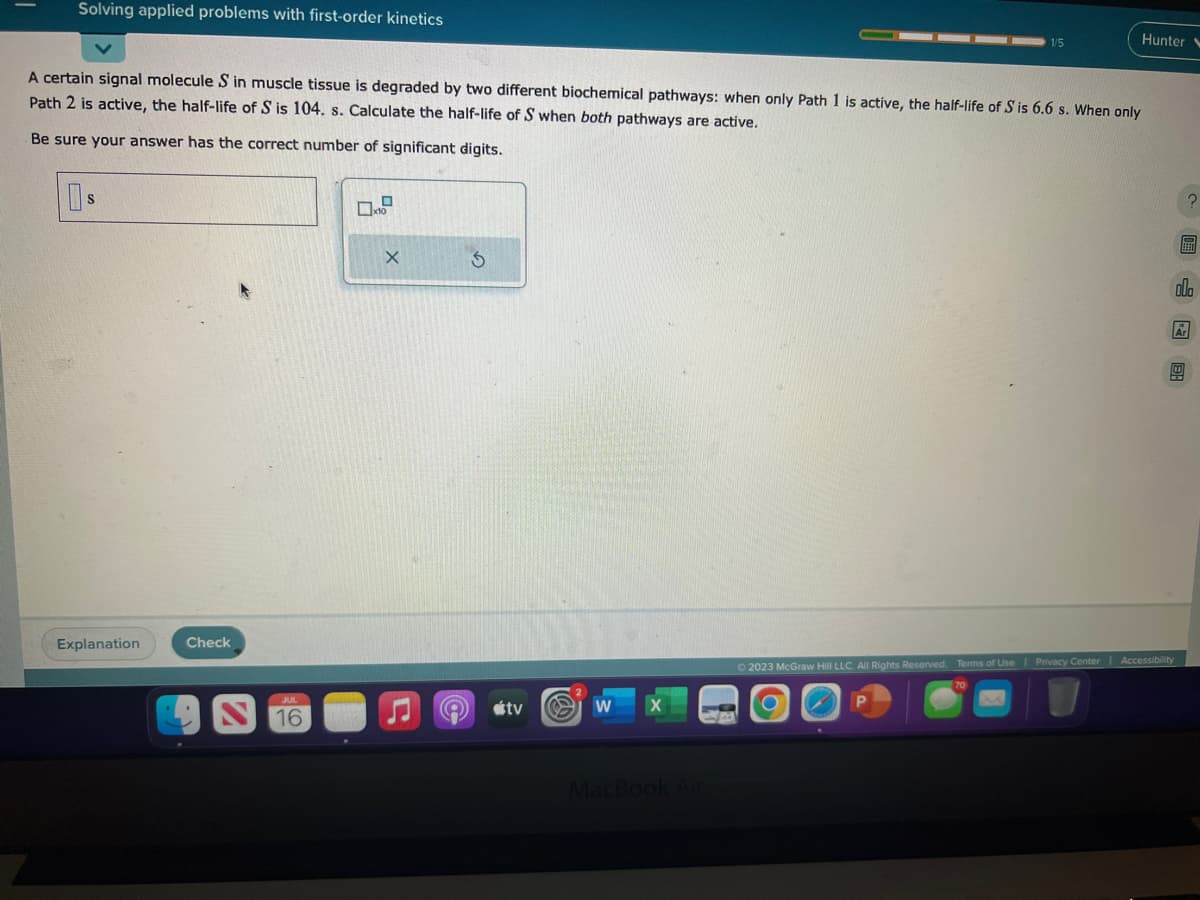
A certain signal molecule S in muscle tissue is degraded by two different biochemical pathways: when only Path 1 is active, the half-life of S is 6.6 s. When only Path 2 is active, the half-life of S is 104. s. Calculate the half-life of S when both pathways are active. Be sure your answer has the correct number of significant digits. 0$ X S Een olo
A certain signal molecule S in muscle tissue is degraded by two different biochemical pathways: when only Path 1 is active, the half-life of S is 6.6 s. When only Path 2 is active, the half-life of S is 104. s. Calculate the half-life of S when both pathways are active. Be sure your answer has the correct number of significant digits. 0$ X S Een olo
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 87QAP: A drug decomposes in the blood by a first-order process. A pill containing 0.500 g of the active...
Related questions
Question
100%
help a girl out???
Transcribed Image Text:Solving applied problems with first-order kinetics
s
A certain signal molecule S in muscle tissue is degraded by two different biochemical pathways: when only Path 1 is active, the half-life of S is 6.6 s. When only
Path 2 is active, the half-life of S is 104. s. Calculate the half-life of S when both pathways are active.
Be sure your answer has the correct number of significant digits.
Explanation
Check
JUL
16
0.0
X
5
9
S
tv
W
1/5
MacBook Air
Hunter V
BE7
Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
olo
Ar
4
Transcribed Image Text:|||
ALEKS Canvas CSU
OKINETICS AND EQUILIBRIUM
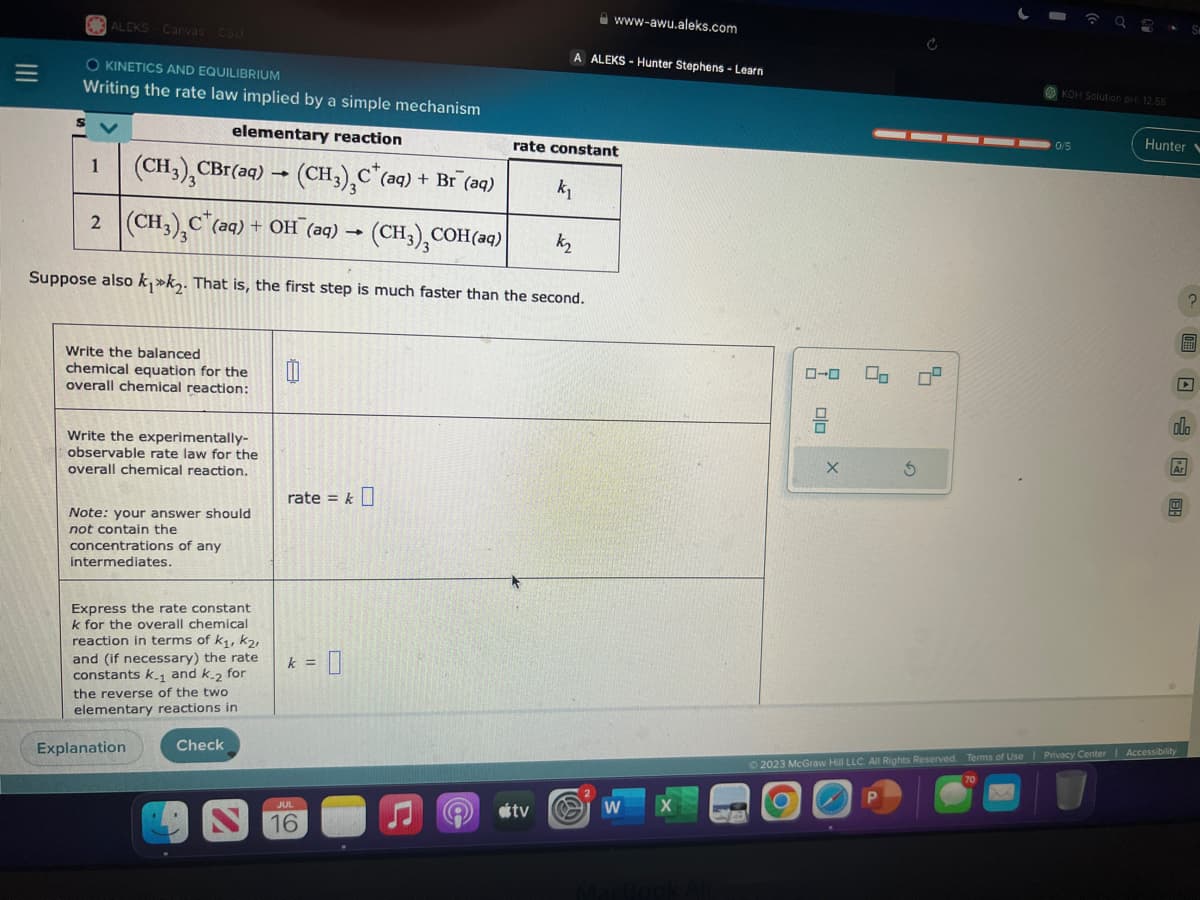
Writing the rate law implied by a simple mechanism
elementary reaction
Write the balanced
chemical equation for the
overall chemical reaction:
1 (CH3), CBr(aq)
(CH3)₂C (aq) + Br (aq)
2 (CH3)₂C (aq) + OH¯(aq) →
(CH3)₂C
k₂
Suppose also k₁k₂. That is, the first step is much faster than the second.
Write the experimentally-
observable rate law for the
overall chemical reaction.
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of K₁, K₂,
and (if necessary) the rate
constants k.₁ and k-2 for
1
the reverse of the two
elementary reactions in
Explanation
Check
-
00
rate = k
k =
JUL
16
COH(aq)
A ALEKS-Hunter Stephens - Learn
rate constant
tv
www-awu.aleks.com
k₁
W
MacBook Air
ローロ
00
X
98
KOH Solution pH: 12.58
0/5
Hunter
n
Ar
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
