A chemist carefully measures the amount of heat needed to raise the temperature of a 576.0 mg sample of a pure substance from -4.3 °C to 20.3 °C. The experiment shows that 10.1 J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the correct number of significant digits. -1 g K
A chemist carefully measures the amount of heat needed to raise the temperature of a 576.0 mg sample of a pure substance from -4.3 °C to 20.3 °C. The experiment shows that 10.1 J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the correct number of significant digits. -1 g K
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 64QAP: The BTU (British thermal unit) is the unit of energy most commonly used in the United States. One...
Related questions
Question
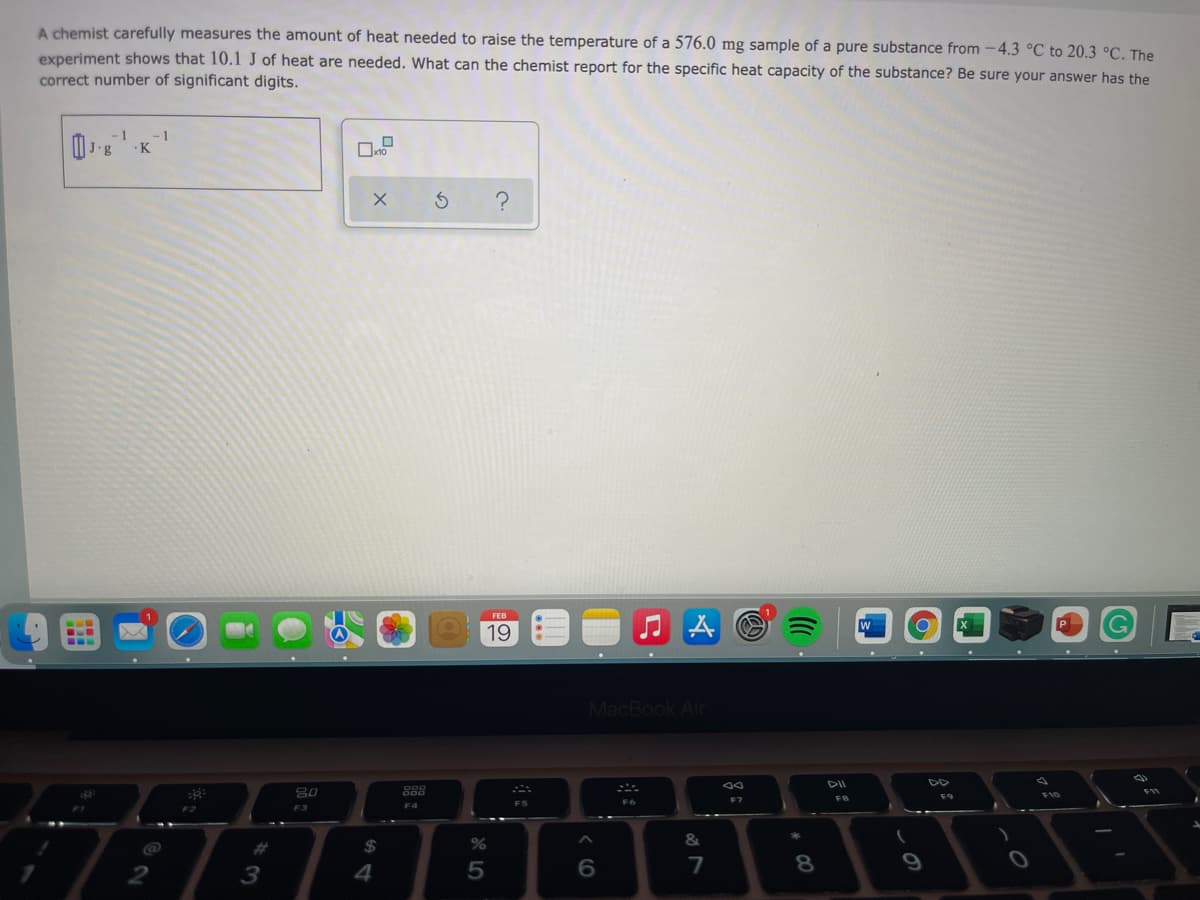
Transcribed Image Text:A chemist carefully measures the amount of heat needed to raise the temperature of a 576.0 mg sample of a pure substance from -4.3 °C to 20.3 °C. The
experiment shows that 10.1 J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the
correct number of significant digits.
-1
-1
·K
W
19
MacBook Air
DD
80
888
F9
F10
F7
FB
F5
F6
F4
F2
F3
&
23
24
5
7
8
Expert Solution
step 1
Given that, for a 576.0 mg of pure substance, 10.1 J of heat is required to raise the temperature from -4.3oC to 20.3oC.
So, the mass of the substance is m = 576.0 mg = 576.010-3 g = 0.576 g.
The heat required is q = 10.1 J.
The initial temperature is T1 = -4.3oC = (273-4.3)K = 268.7K.
The final temperature is T2 = 20.3oC = (273+20.3)K = 293.3K.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning