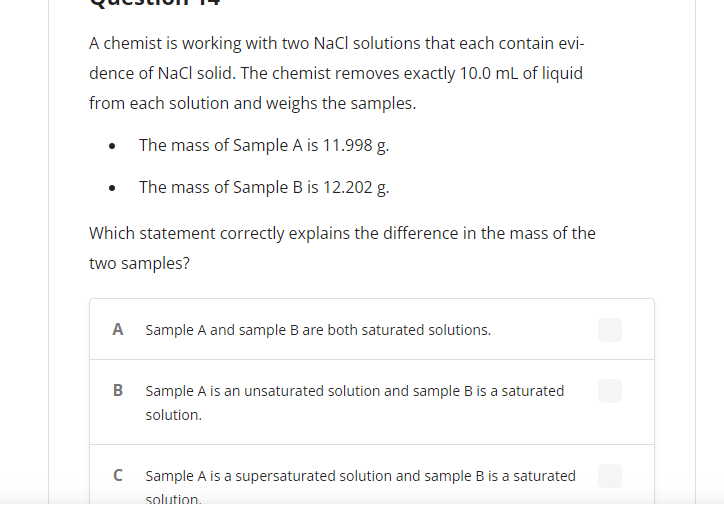
A chemist is working with two NaCl solutions that each contain evi- dence of NaCl solid. The chemist removes exactly 10.0 mL of liquid from each solution and weighs the samples. The mass of Sample A is 11.998 g. The mass of Sample B is 12.202 g. Which statement correctly explains the difference in the mass of the two samples? A Sample A and sample B are both saturated solutions. B Sample A is an unsaturated solution and sample B is a saturated solution. Sample A is a supersaturated solution and sample B is a saturated solution.
A chemist is working with two NaCl solutions that each contain evi- dence of NaCl solid. The chemist removes exactly 10.0 mL of liquid from each solution and weighs the samples. The mass of Sample A is 11.998 g. The mass of Sample B is 12.202 g. Which statement correctly explains the difference in the mass of the two samples? A Sample A and sample B are both saturated solutions. B Sample A is an unsaturated solution and sample B is a saturated solution. Sample A is a supersaturated solution and sample B is a saturated solution.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 9E: What happens if you add a very small amount of solid salt (NaCl) to each beaker described below?...
Related questions
Question
Transcribed Image Text:A chemist is working with two NaCl solutions that each contain evi-
dence of NaCl solid. The chemist removes exactly 10.0 mL of liquid
from each solution and weighs the samples.
The mass of Sample A is 11.998 g.
The mass of Sample B is 12.202 g.
Which statement correctly explains the difference in the mass of the
two samples?
A Sample A and sample B are both saturated solutions.
B Sample A is an unsaturated solution and sample B is a saturated
solution.
C
Sample A is a supersaturated solution and sample B is a saturated
solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning