(b) The ring opening of epoxides (oxirancs) with thiols is catalysed by sodium hydroxide in aqueous media at 300 K. For a typical reaction, the pH-rate profile, between pH 8 and 14 is linear with a slope of +1. What type of catalysis is involved here - give an appropriate rcaction mechanism and state how you would establish the numerical value of the second order rate-constant for the process from the pH-rate profile. Your answer should include a definition of the term pH-rate profile and a statement of the accompanying rate equation for the process. R'-SH NaOH OH R2 Water R1-S R2
(b) The ring opening of epoxides (oxirancs) with thiols is catalysed by sodium hydroxide in aqueous media at 300 K. For a typical reaction, the pH-rate profile, between pH 8 and 14 is linear with a slope of +1. What type of catalysis is involved here - give an appropriate rcaction mechanism and state how you would establish the numerical value of the second order rate-constant for the process from the pH-rate profile. Your answer should include a definition of the term pH-rate profile and a statement of the accompanying rate equation for the process. R'-SH NaOH OH R2 Water R1-S R2
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter8: Haloalkanes, Halogenation, And Radical Reactions
Section: Chapter Questions
Problem 8.34P
Related questions
Question
2
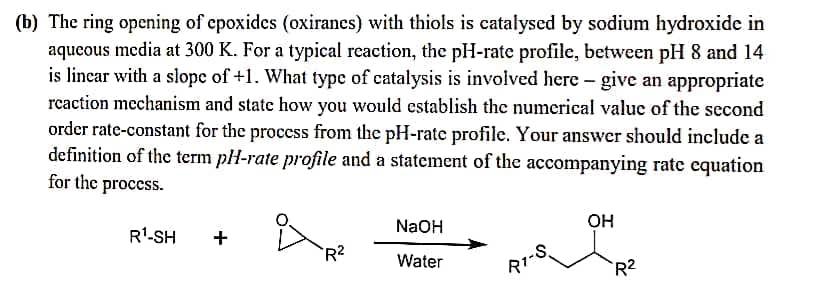
Transcribed Image Text:(b) The ring opening of epoxides (oxiranes) with thiols is catalysed by sodium hydroxide in
aqueous media at 300 K. For a typical reaction, the pH-rate profile, between pH 8 and 14
is linear with a slope of +1. What type of catalysis is involved here - give an appropriate
rcaction mechanism and state how you would establish the numerical value of the second
order rate-constant for the proccss from the pH-rate profile. Your answer should include a
definition of the term pH-rate profile and a statement of the accompanying rate equation
for the process.
NaOH
OH
R'-SH
+
-S.
R1
R2
Water
R2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning