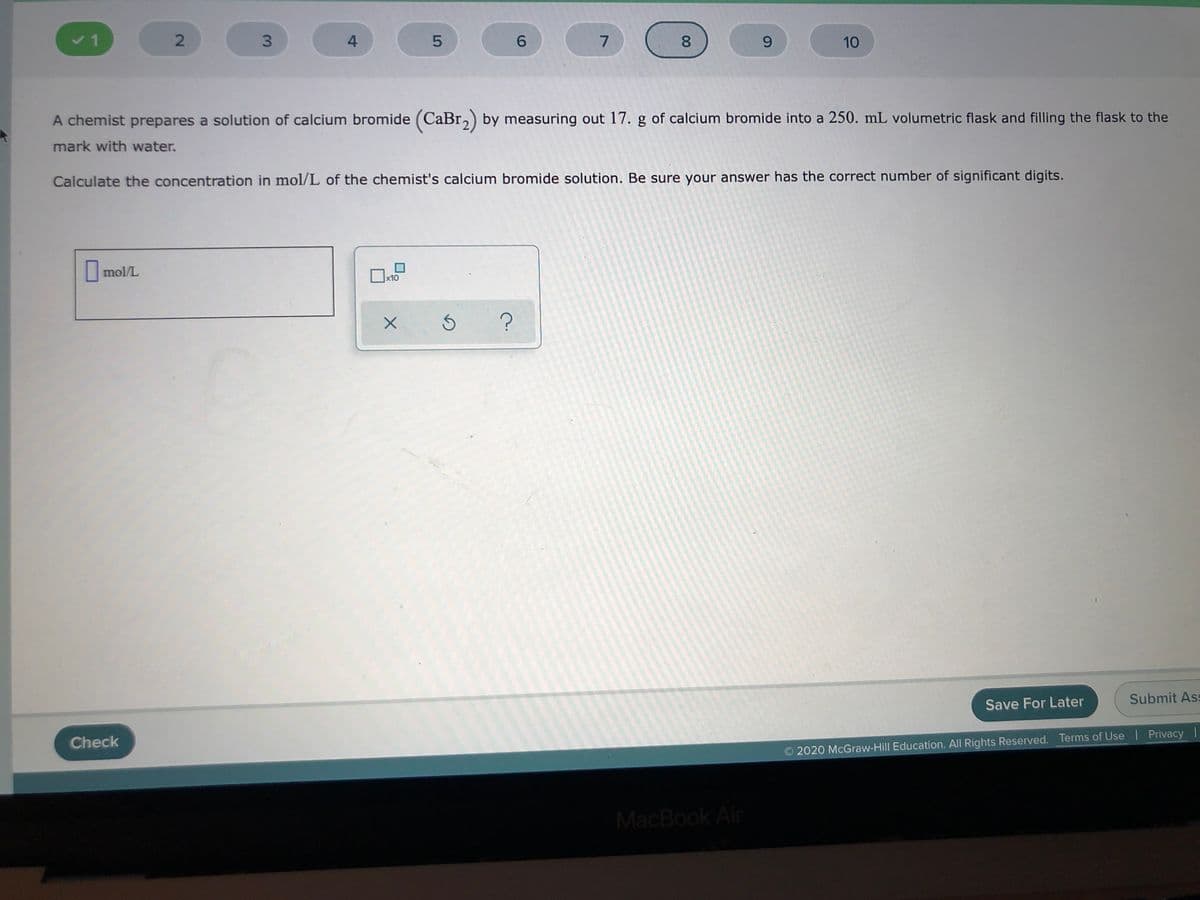
A chemist prepares a solution of calcium bromide (CaBr,) by measuring out 17. g of calcium bromide into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's calcium bromide solution. Be sure your answer has the correct number of significant digits.
A chemist prepares a solution of calcium bromide (CaBr,) by measuring out 17. g of calcium bromide into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's calcium bromide solution. Be sure your answer has the correct number of significant digits.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
Transcribed Image Text:2
3.
4.
6.
8.
10
v1
A chemist prepares a solution of calcium bromide (CaBr,) by measuring out 17. g of calcium bromide into a 250. mL volumetric flask and filling the flask to the
mark with water.
Calculate the concentration in mol/L of the chemist's calcium bromide solution. Be sure your answer has the correct number of significant digits.
mol/L
x10
?
Submit Ass
Save For Later
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy I
MacBook Air
Transcribed Image Text:6.
9.
10
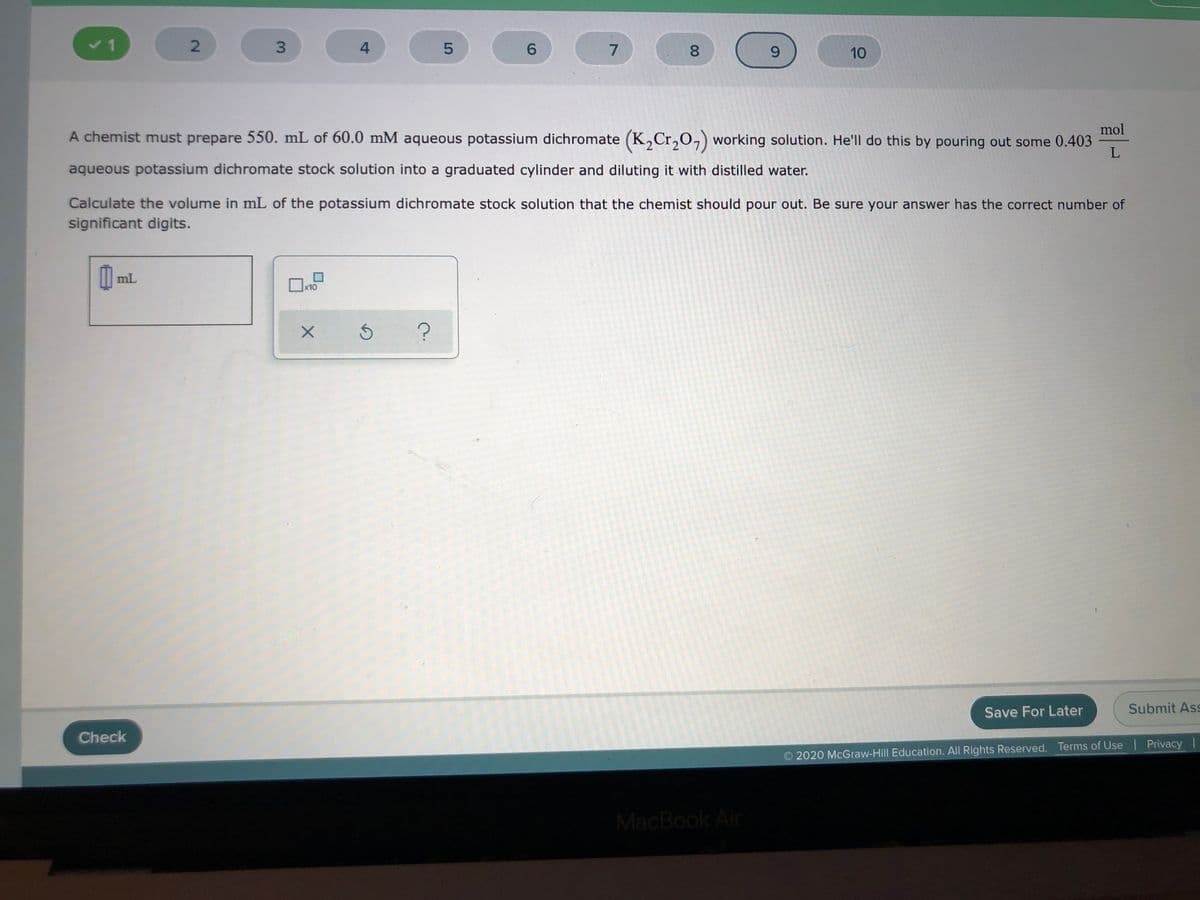
mol
A chemist must prepare 550. mL of 60.0 mM aqueous potassium dichromate (K,Cr,O,) working solution. He'll do this by pouring out some 0.403
aqueous potassium dichromate stock solution into a graduated cylinder and diluting it with distilled water.
Calculate the volume in mL of the potassium dichromate stock solution that the chemist should pour out. Be sure your answer has the correct number of
significant digits.
I| mL
x10
Save For Later
Submit Ass
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy I
MacBook Air
00
4-
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



