A chemistry student adds a quantity of an unknown solid compound X to 600. mL of distilled water at 25.° C. After 10 minutes of stirring, only a little X has dissolved. The student then drains off the solution and evaporates the water under vacuum. A precipitate is left behind. The student washes, dries and weighs the precipitate. It weighs 48.0 g. yes Using only the information above, can you calculate the solubility of X in water at 25.° C ? no If you said yes, calculate it. Be sure your answer has a unit symbol and 3 significant digits. c.
A chemistry student adds a quantity of an unknown solid compound X to 600. mL of distilled water at 25.° C. After 10 minutes of stirring, only a little X has dissolved. The student then drains off the solution and evaporates the water under vacuum. A precipitate is left behind. The student washes, dries and weighs the precipitate. It weighs 48.0 g. yes Using only the information above, can you calculate the solubility of X in water at 25.° C ? no If you said yes, calculate it. Be sure your answer has a unit symbol and 3 significant digits. c.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 31E: Common commercial acids and bases are aqueous solutions with the following properties: Density...
Related questions
Question
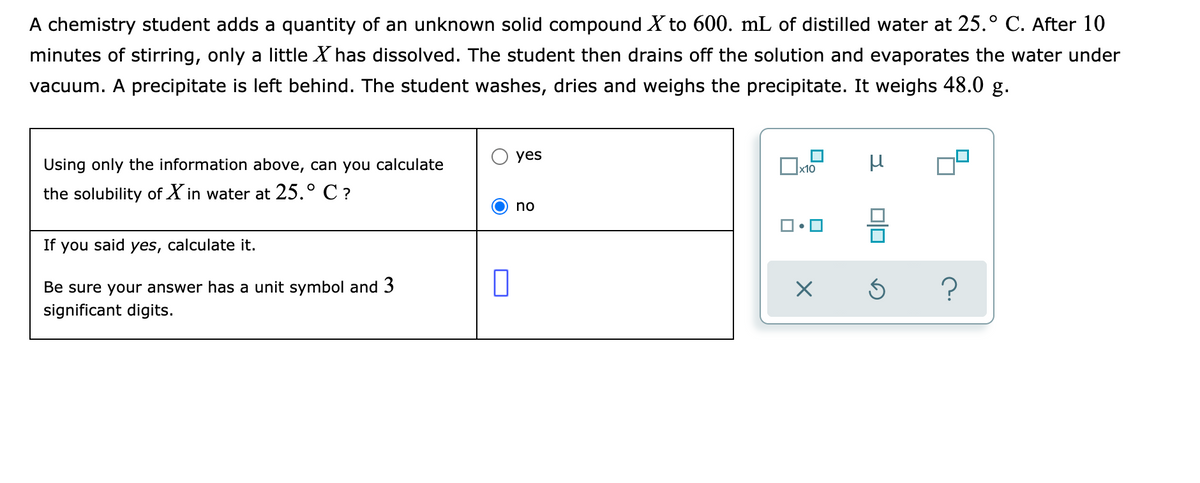
Transcribed Image Text:A chemistry student adds a quantity of an unknown solid compound X to 600. mL of distilled water at 25.° C. After 10
minutes of stirring, only a little X has dissolved. The student then drains off the solution and evaporates the water under
vacuum. A precipitate is left behind. The student washes, dries and weighs the precipitate. It weighs 48.0 g.
yes
Using only the information above, can you calculate
the solubility of X in water at 25.° C ?
no
If you said yes, calculate it.
Be sure your answer has a unit symbol and 3
significant digits.
미
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning