Dissection х-ахis у-ахis Solubility (g Pb(NO3)2/100 g water) Plot below points and Draw a line between the points Temperature (°C) and beyond in both directions past the data points. Use this line to answer the following questions. 92.1 115 1. What would the solubility be at 35 °C of lead 70.1 91.2 nitrate? 49.6 78.6 2. What would the solubility be at 25 °C of lead 31.2 61.4 nitrate? 3. What temperature would be necessary to create a solution with solubility of 65 g/100 ml?
Dissection х-ахis у-ахis Solubility (g Pb(NO3)2/100 g water) Plot below points and Draw a line between the points Temperature (°C) and beyond in both directions past the data points. Use this line to answer the following questions. 92.1 115 1. What would the solubility be at 35 °C of lead 70.1 91.2 nitrate? 49.6 78.6 2. What would the solubility be at 25 °C of lead 31.2 61.4 nitrate? 3. What temperature would be necessary to create a solution with solubility of 65 g/100 ml?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 7RQ
Related questions
Question
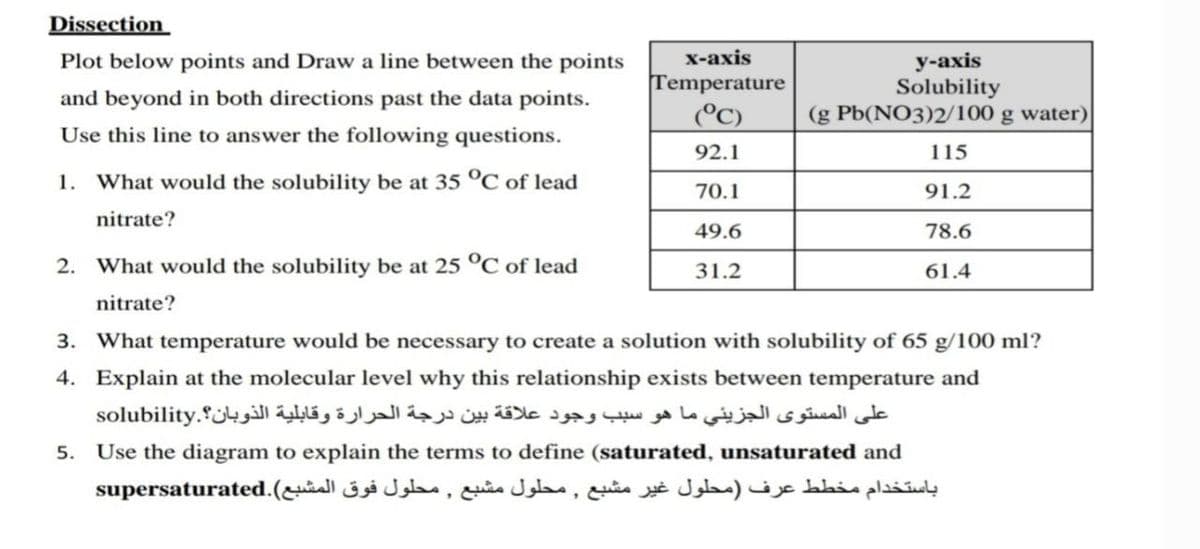
Transcribed Image Text:Dissection
х-ахis
у-аxis
Solubility
(g Pb(NO3)2/100 g water)
Plot below points and Draw a line between the points
Temperature
(°C)
and beyond in both directions past the data points.
Use this line to answer the following questions.
92.1
115
1. What would the solubility be at 35 °C of lead
70.1
91.2
nitrate?
49.6
78.6
2. What would the solubility be at 25 °C of lead
31.2
61.4
nitrate?
3. What temperature would be necessary to create a solution with solubility of 65 g/100 ml?
4. Explain at the molecular level why this relationship exists between temperature and
على المستوى الجزيئي ما هو سبب وجود علاقة بين درجة الحرارة وقابلية الذوبان؟.solubility
5.
Use the diagram to explain the terms to define (saturated, unsaturated and
باستخدام مخطط عرف )محلول غیر مشبع , محلول مشبع , محلول فوق المشبع(.supersaturated
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning