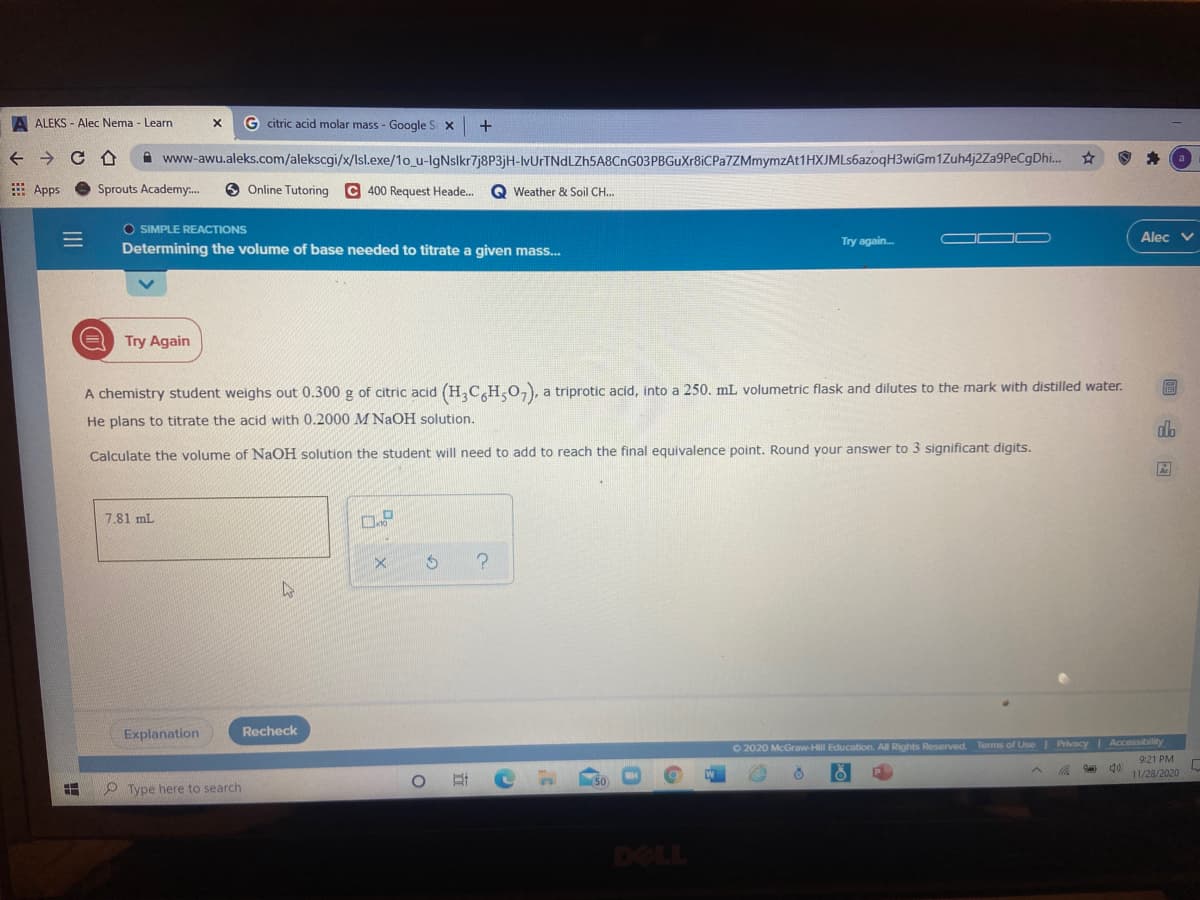
A chemistry student weighs out 0.300 g of citric acid (H,C H,O,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.2000 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits. 7.81 mL |回山因
A chemistry student weighs out 0.300 g of citric acid (H,C H,O,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.2000 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits. 7.81 mL |回山因
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.76P
Related questions
Question
100%
Transcribed Image Text:A ALEKS - Alec Nema - Learm
G citric acid molar mass - Google S x
A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmymzAt1HXJMLs6azoqH3wiGm1Zuh4j2Za9PeCgDhi..
E Apps
Sprouts Academy.
O Online Tutoring C 400 Request Heade..
Q Weather & Soil C.
O SIMPLE REACTIONS
Try again.
Alec v
Determining the volume of base needed to titrate a given mass..
Try Again
A chemistry student weighs out 0.300 g of citric acid (H,C H,0,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water.
He plans to titrate the acid with 0.2000 M NAOH solution.
dlo
Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.
7.81 mL
Explanation
Recheck
O 2020 McGrow-Hill Education. All Rights Reserved Terms of Use Privacy Accessibility
9:21 PM
11/28/2020
50
O Type here to search
DELL
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning