A chemist must prepare 800. mL of 69.0 µM aqueous mercury(II) iodide (HgI,) working solution. He'll do this by pouring out some 0.000101 M aqueous mercury(II) iodide stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the mercury(II) iodide stock solution that the chemist should pour out. Round your answer to 3 significant digits.
A chemist must prepare 800. mL of 69.0 µM aqueous mercury(II) iodide (HgI,) working solution. He'll do this by pouring out some 0.000101 M aqueous mercury(II) iodide stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the mercury(II) iodide stock solution that the chemist should pour out. Round your answer to 3 significant digits.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 62QAP
Related questions
Question
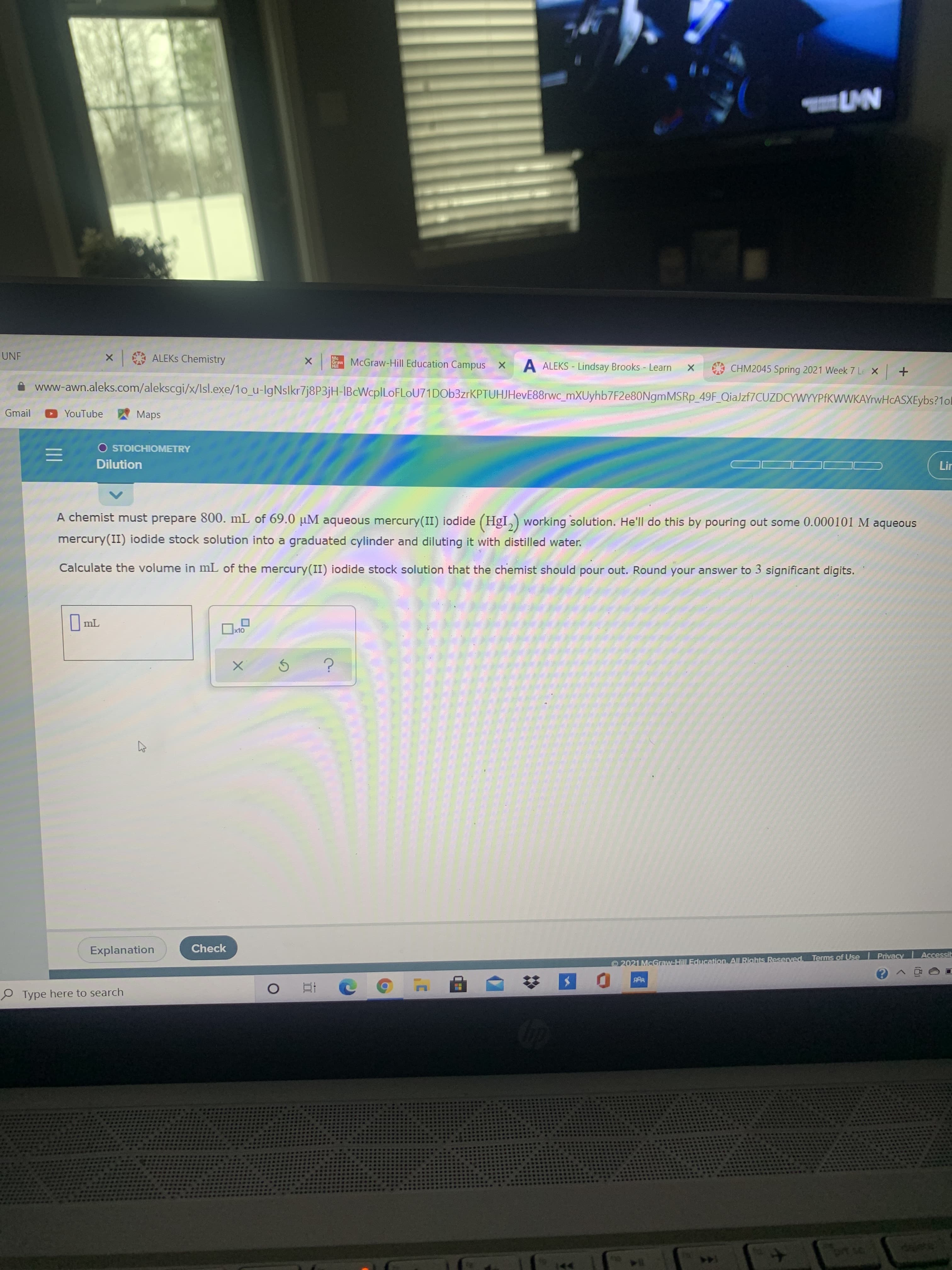
Transcribed Image Text:A chemist must prepare 800. mL of 69.0 µM aqueous mercury(II) iodide (HgI,) working solution. He'll do this by pouring out some 0.000101 M aqueous
mercury(II) iodide stock solution into a graduated cylinder and diluting it with distilled water.
Calculate the volume in mL of the mercury(II) iodide stock solution that the chemist should pour out. Round your answer to 3 significant digits.
Expert Solution
Step 1
Given : Volume of solution to be prepared = 800 mL
Concentration of HgI2 = 69.0 µM = 69.0 X 10-6 M (since 1 M = 106 µM )
And concentration of HgI2 stock solution = 0.000101 M
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
