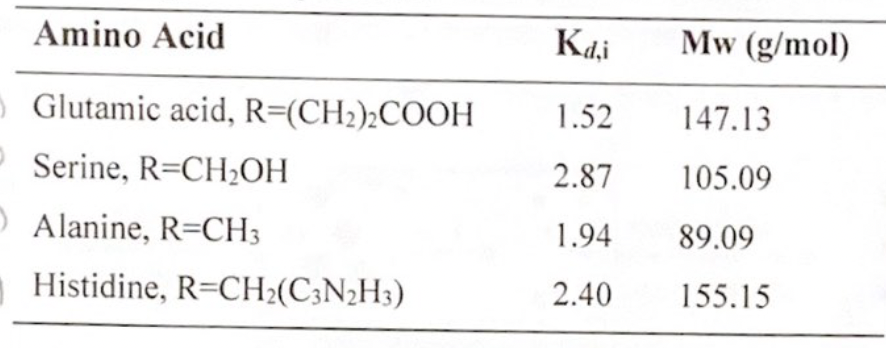
A chromatography column is used to separate four amino acids: glutamic acid, serine, alanine, and histidine. A cylindrical column is packed (65% by volume) with nonporous polymer particles. An aqueous solution containing 25 mol % of each amino acid is fed into the column with a superficial solution velocity of 0.32 mm/sec) The column is operated continuously using a 550 sec feed (pulse) time followed by elusion for a set period of time. Given the equilibrium constants below for each amino acid, answer the following sets of questions: A. What is the minimum length of the column required to completely separate the amino acids? B. What is the time required for solvent elution (time for all amino acids to exit the column)?
A chromatography column is used to separate four amino acids: glutamic acid, serine, alanine, and histidine. A cylindrical column is packed (65% by volume) with nonporous polymer particles. An aqueous solution containing 25 mol % of each amino acid is fed into the column with a superficial solution velocity of 0.32 mm/sec) The column is operated continuously using a 550 sec feed (pulse) time followed by elusion for a set period of time. Given the equilibrium constants below for each amino acid, answer the following sets of questions:
A. What is the minimum length of the column required to completely separate the amino acids?
B. What is the time required for solvent elution (time for all amino acids to exit the column)?
Step by step
Solved in 3 steps with 2 images









