A cloud is observed to commence formation at a temperature of −61° C and continues to cool due to emission of infrared radiation. a) Determine the partial pressure (Pa) and density of water vapour (mg/m³) in the air before cloud formation commenced. b) Determine the partial pressure (Pa) and density of water vapour (mg/m³) in the cloud when the temperature is -63° C. c) Calculate the ice water content (mg/m³) in the cloud when the temperature is −63º C.
A cloud is observed to commence formation at a temperature of −61° C and continues to cool due to emission of infrared radiation. a) Determine the partial pressure (Pa) and density of water vapour (mg/m³) in the air before cloud formation commenced. b) Determine the partial pressure (Pa) and density of water vapour (mg/m³) in the cloud when the temperature is -63° C. c) Calculate the ice water content (mg/m³) in the cloud when the temperature is −63º C.
Applications and Investigations in Earth Science (9th Edition)
9th Edition
ISBN:9780134746241
Author:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Chapter1: The Study Of Minerals
Section: Chapter Questions
Problem 1LR
Related questions
Question
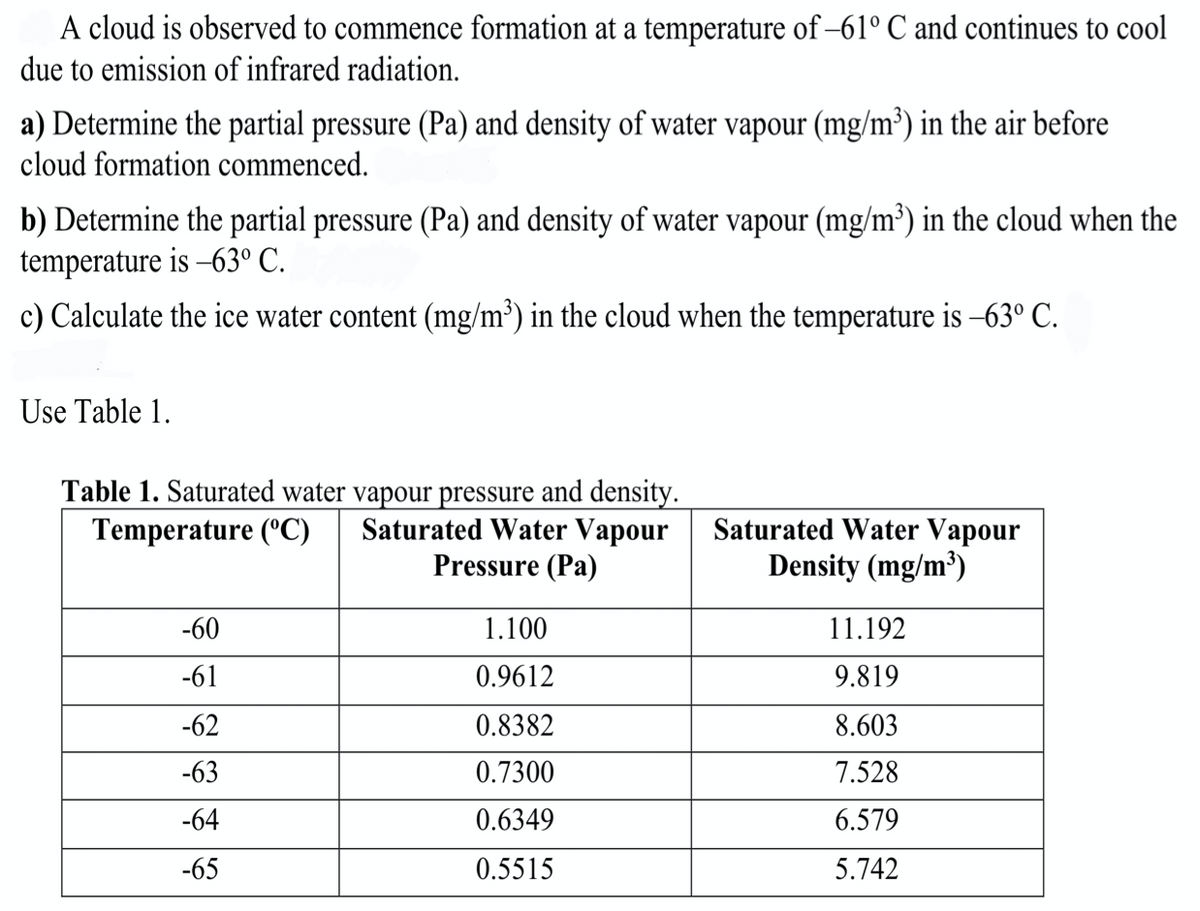
Transcribed Image Text:A cloud is observed to commence formation at a temperature of-61° C and continues to cool
due to emission of infrared radiation.
a) Determine the partial pressure (Pa) and density of water vapour (mg/m³) in the air before
cloud formation commenced.
b) Determine the partial pressure (Pa) and density of water vapour (mg/m³) in the cloud when the
temperature is -63° C.
c) Calculate the ice water content (mg/m³) in the cloud when the temperature is −63° C.
Use Table 1.
Table 1. Saturated water vapour pressure and density.
Temperature (°C)
Saturated Water Vapour
Pressure (Pa)
-60
-61
-62
-63
-64
-65
1.100
0.9612
0.8382
0.7300
0.6349
0.5515
Saturated Water Vapour
Density (mg/m³)
11.192
9.819
8.603
7.528
6.579
5.742
![Wien's displacement Law: Ap=2898/Tum
Kirchoff's Law: absorptivity = emissivity
Flux of solar Radiation at Earth: F, = T1370 Watts/m²
Effective Radiating Temperature for the Earth (current climate): T₂ = 255 K
Beer's Law: 1(x) = 1(0) exp[-x]; x = kpdx = fondx
Optical cross section: k in
Force of buoyancy: Fg = g
kg
Ideal Gas Law: P = pRT; R = 287 J Kg¹ K
Ideal Gas Law: P = nkT; k=1.381x 1023 J/K
Ideal Gas Law for Water Vapour: e=p, R, T; R = 461.5 J Kg¹ K-¹
Hydrostatic Equation:=-pg
Barometric Law: P(z) = P(0) exp(-); H = RT/g; P. = 100 × 10³ Pa; g = 9.81 m/s²
(Po-P)
(T-T₂)
P
To
dT
Adiabatic Lapse Rate:
dz
or o in-
m²
molecule
Cp
= g
First Law of Thermodynamics: &q= c₂ dT + P da or 8q = cp dT - 1 dp
Specific Heat Capacity for Air: c=1005;
CAPER
-9.8 °C/km
EL
So
LFC
Potential Temperature: 0 = T
Brunt-Vaisala Frequency, or Buoyancy Frequency: N² = 98
=
0 dz
Latent Heat of Condensation for water: 2535 J/g
Latent Heat of Sublimation for water: 2834 J/g
Les
Clausius-Clapeyron Equation: dT
R₂T²
Solution to C-C equation: ()
eso
Joules
kg-deg C
= exp
{ (- Đ
Saturated (Wet) Adiabatic Lapse Rate: I'
at To 0 °C (273 K), eso = 611 Pa
Equivalent Potential Temperature: 0 = 0 exp
Adiabatic liquid/ice water content: x = -
Velocity: v² v² = 2a(z-zo); a is acceleration
(T-T₂) dinPo
dT
dz
LWS
1+
L dws
Cp dr
Information (as provided on 2021 final exam)
Earth-Sun mean distance: 149.598 x 10⁹ m
Radius of Sun: 6.96 x 10³ m
Radius of Earth: 6371 x 10³ m
Effective temperature of Sun: 5770 K
Cross sectional area of a sphere: R²
Surface area of a sphere: 4R²
Solid Angle: 2 = Area on sphere/R²; d = sine de do
Albedo of the Earth: A = 0.3
Plank Function: B(A,T) =
2hc²
hc
As ekXT-1
Plank's constant: h = 6.626 × 10-³4 Js
Boltzmann's constant: k = 1.381x 1023 J/K
Speed of light: c = 3 x 10³ m/s
Flux: F = f I cose d
Watts
m² μm-ST
Watts
m²
Stephan's constant: = 5.67 × 108 Wm² K-4
Net flux upward or downward for isotropic radiation: F = al
Stephan-Boltzmann Law: F = 6T4
-Aws(); or x = - Δρ. (mg)
kg
m³](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd28de0e5-9c94-4bd5-a9be-fdd69ffbee6a%2F23fe84aa-938a-4dcf-a6d9-f23059e99712%2Fvq76kzb_processed.png&w=3840&q=75)
Transcribed Image Text:Wien's displacement Law: Ap=2898/Tum
Kirchoff's Law: absorptivity = emissivity
Flux of solar Radiation at Earth: F, = T1370 Watts/m²
Effective Radiating Temperature for the Earth (current climate): T₂ = 255 K
Beer's Law: 1(x) = 1(0) exp[-x]; x = kpdx = fondx
Optical cross section: k in
Force of buoyancy: Fg = g
kg
Ideal Gas Law: P = pRT; R = 287 J Kg¹ K
Ideal Gas Law: P = nkT; k=1.381x 1023 J/K
Ideal Gas Law for Water Vapour: e=p, R, T; R = 461.5 J Kg¹ K-¹
Hydrostatic Equation:=-pg
Barometric Law: P(z) = P(0) exp(-); H = RT/g; P. = 100 × 10³ Pa; g = 9.81 m/s²
(Po-P)
(T-T₂)
P
To
dT
Adiabatic Lapse Rate:
dz
or o in-
m²
molecule
Cp
= g
First Law of Thermodynamics: &q= c₂ dT + P da or 8q = cp dT - 1 dp
Specific Heat Capacity for Air: c=1005;
CAPER
-9.8 °C/km
EL
So
LFC
Potential Temperature: 0 = T
Brunt-Vaisala Frequency, or Buoyancy Frequency: N² = 98
=
0 dz
Latent Heat of Condensation for water: 2535 J/g
Latent Heat of Sublimation for water: 2834 J/g
Les
Clausius-Clapeyron Equation: dT
R₂T²
Solution to C-C equation: ()
eso
Joules
kg-deg C
= exp
{ (- Đ
Saturated (Wet) Adiabatic Lapse Rate: I'
at To 0 °C (273 K), eso = 611 Pa
Equivalent Potential Temperature: 0 = 0 exp
Adiabatic liquid/ice water content: x = -
Velocity: v² v² = 2a(z-zo); a is acceleration
(T-T₂) dinPo
dT
dz
LWS
1+
L dws
Cp dr
Information (as provided on 2021 final exam)
Earth-Sun mean distance: 149.598 x 10⁹ m
Radius of Sun: 6.96 x 10³ m
Radius of Earth: 6371 x 10³ m
Effective temperature of Sun: 5770 K
Cross sectional area of a sphere: R²
Surface area of a sphere: 4R²
Solid Angle: 2 = Area on sphere/R²; d = sine de do
Albedo of the Earth: A = 0.3
Plank Function: B(A,T) =
2hc²
hc
As ekXT-1
Plank's constant: h = 6.626 × 10-³4 Js
Boltzmann's constant: k = 1.381x 1023 J/K
Speed of light: c = 3 x 10³ m/s
Flux: F = f I cose d
Watts
m² μm-ST
Watts
m²
Stephan's constant: = 5.67 × 108 Wm² K-4
Net flux upward or downward for isotropic radiation: F = al
Stephan-Boltzmann Law: F = 6T4
-Aws(); or x = - Δρ. (mg)
kg
m³
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Earth Science (15th Edition)
Earth Science
ISBN:
9780134543536
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Environmental Science (MindTap Course List)
Earth Science
ISBN:
9781337569613
Author:
G. Tyler Miller, Scott Spoolman
Publisher:
Cengage Learning

Physical Geology
Earth Science
ISBN:
9781259916823
Author:
Plummer, Charles C., CARLSON, Diane H., Hammersley, Lisa
Publisher:
Mcgraw-hill Education,