A common demonstration in chemistry courses involves adding a tiny speck of manganese(IV) oxide to a concentrated hydro- gen peroxide (H₂O₂) solution. Hydrogen peroxide decomposes quite spectacularly under these conditions to produce oxygen gas and steam (water vapor). Manganese(IV) oxide is a catalyst for the decomposition of hydrogen peroxide and is not consumed in the reaction. Write the balanced equation for the decomposition reaction of hydrogen peroxide. 92. Iron oxide ores, commonly a mixture of FeO and Fe,O,, are given the general formula Fe,O,. They yield elemental iron when heated to a very high temperature with either carbon monoxide or elemen- tal hydrogen. Balance the following equations for these processes: Fe,O(s) + H₂(g) Fe 0,(s) + CO(e) Fe(s) + H₂O(e) Fe(s) + CO)
A common demonstration in chemistry courses involves adding a tiny speck of manganese(IV) oxide to a concentrated hydro- gen peroxide (H₂O₂) solution. Hydrogen peroxide decomposes quite spectacularly under these conditions to produce oxygen gas and steam (water vapor). Manganese(IV) oxide is a catalyst for the decomposition of hydrogen peroxide and is not consumed in the reaction. Write the balanced equation for the decomposition reaction of hydrogen peroxide. 92. Iron oxide ores, commonly a mixture of FeO and Fe,O,, are given the general formula Fe,O,. They yield elemental iron when heated to a very high temperature with either carbon monoxide or elemen- tal hydrogen. Balance the following equations for these processes: Fe,O(s) + H₂(g) Fe 0,(s) + CO(e) Fe(s) + H₂O(e) Fe(s) + CO)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 97E
Related questions
Question
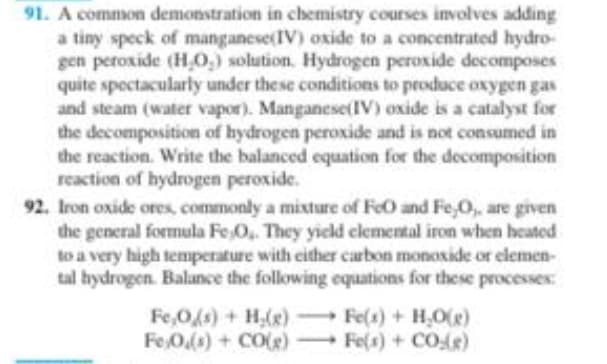
Transcribed Image Text:91. A common demonstration in chemistry courses involves adding
a tiny speck of manganese(IV) oxide to a concentrated hydro-
gen peroxide (H₂O₂) solution. Hydrogen peroxide decomposes
quite spectacularly under these conditions to produce oxygen gas
and steam (water vapor). Manganese(IV) oxide is a catalyst for
the decomposition of hydrogen peroxide and is not consumed in
the reaction. Write the balanced equation for the decomposition
reaction of hydrogen peroxide.
92. Iron oxide ores, commonly a mixture of FeO and Fe,O,, are given
the general formula Fe,O,. They yield elemental iron when heated
to a very high temperature with either carbon monoxide or elemen-
tal hydrogen. Balance the following equations for these processes:
Fe,O(s) + H₂(g) →→→
Fe 0.(s) + CO(e)
Fe(s) + H₂O(e)
Fe(s) + CO)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning