on the right. 1s 2s 2p 3s 3p 4s²3d¹04p5s²4d¹05p 1s 2s 2p 3s 3p 4s²3d¹04p5s²4d¹05p6s 1s 2s 2p 3s 3p 4s³3d¹04p Submit Anwe 1s 2s 2p 3s 3p5 Ratry Endre Grove 20 Clear All group attempts remaining X.
on the right. 1s 2s 2p 3s 3p 4s²3d¹04p5s²4d¹05p 1s 2s 2p 3s 3p 4s²3d¹04p5s²4d¹05p6s 1s 2s 2p 3s 3p 4s³3d¹04p Submit Anwe 1s 2s 2p 3s 3p5 Ratry Endre Grove 20 Clear All group attempts remaining X.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 1E: Write the electronic configuration for the ions of the third period element that form monoatomic...
Related questions
Question
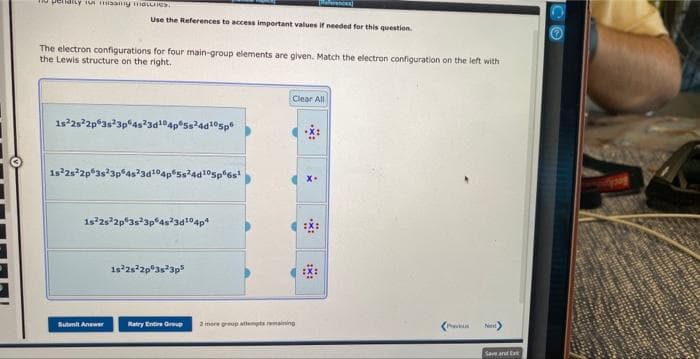
Transcribed Image Text:o periarty for missiy moles
The electron configurations for four main-group elements are given. Match the electron configuration on the left with
the Lewis structure on the right.
Use the References to access important values if needed for this question.
1s²2s²2p 3s 3p 4s²3d¹04p 5s²4d¹05p
1s22s22p 3s 3p
Sutmil Anew
Ss24d105p6s¹
1s 2s 2p 3s 3p 4s23d¹04p4
1s²2s22p 3s 3p5
Ratry Entire Group
Clear All
2 inore group attempts remaining
:x:
Next
Save and Ext
CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning