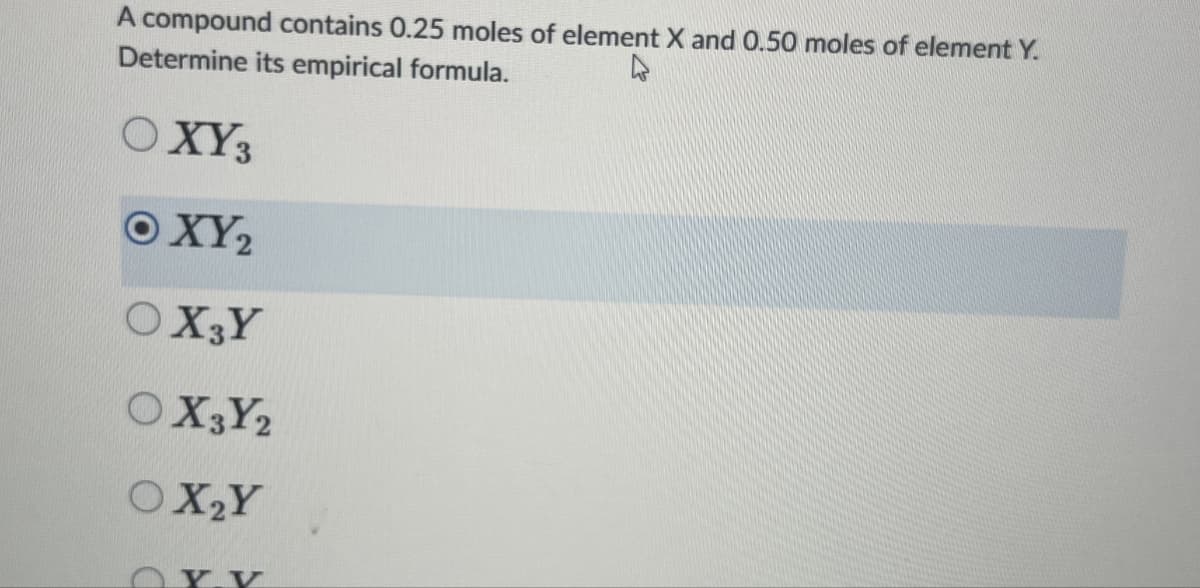
A compound contains 0.25 moles of element X and 0.50 moles of element Y. Determine its empirical formula. OXY3 ©XY, OX3Y OX3Y2 OX2Y
Q: Rahul
A: In accounting and finance we use regression analysis to determine the relationship between the…
Q: is the below correct? Requirement:-is statement correct? Statement of Profit or Loss For the period…
A: Now we can dissect the statement further: First, income: During the stipulated period, the business…
Q: https://massygroup.com/wp-content/uploads/2022/11/MASSY-DIGITAL-ANNUAL- REPORT-2022-updated.pdf a.…
A: To analyze Massy Holdings Ltd.'s financial performance and its implications for shareholders,…
Q: x M Question 3-QUIZ- CH 17-C X Chapter 5: Customers and Sal x +…
A: Step 1: Given costs as % of sales: production level support BeforeAftervariable manufacturing…
Q: A business issued a 30-day note for $57,000 to a bank. The note was discounted at 7%. Assume a 360…
A: The objective of this question is to understand how to journalize the issuance and payment of a…
Q: You are considering a new product launch. The project will cost $1,500,000, have a four- year life,…
A: New Product Launch Analysisa-1. Sensitivity Analysis (Unit ±10%)Upper and Lower Bounds:Unit…
Q: Valmont Company developed a new industrial piece of equipment called the XP-200. The company is…
A: 1. If Valmont uses absorption cost-plus pricing, the price it will establish for the XP-200 can be…
Q: Question 5 The XYZ Partnership is being formed by three partners. Their ownership in the…
A: The objective of the question is to determine the tax year that the XYZ Partnership can elect…
Q: solve using excel
A: Part 2:Explanation:Step 1: Calculate the total number of units available for sale in…
Q: Allowance for Doubtful Accounts has a debit balance of $830 before adjustment. (Credit account…
A: The adjustment of the Allowance for Doubtful Accounts involves recognizing the estimated portion of…
Q: Huron Company produces a commercial cleaning compound known as Zoom. The direct materials and direct…
A:
Q: The payroll register for D. Salah Company for the week ended May 18 indicated the following: Line…
A: a. Journalize the entry to record the payroll for the week of May 18.Date Account Debit CreditMay 18…
Q: Real Estate The following table shows the cost of 1,000 square feet of luxury real estate in three…
A:
Q: 6. Baker Co. provides the following information related to its pension plan for the year 2022: a.…
A: A pension plan is a retirement savings plan sponsored by an employer that allows employees to save a…
Q: A shareholder owns 45% of a S corporation stock for 175 days and 40% of the S corporation's stock…
A: The objective of the question is to calculate the shareholder's share of the ordinary income of the…
Q: 2. Date The following information relates to the Windsor Company. Ending Inventory Price…
A: The dollar-value LIFO (Last In, First Out) method is a technique used by companies to account for…
Q: You are a financial accountant for a small business and have been tasked with preparing a monograph…
A: In the month of our scenario, ABC Mart, a fictional retail store, experienced various economic…
Q: please explain your answer
A:
Q: Prepare only the Property, plant and equipment reconciliation note of Still Waters Ltd for the…
A: Fixed assetsThe assets available in the company for the use of operating activities and used for a…
Q: (b) Your answer is correct. Calculate the days in inventory for 2020, 2021, and 2022. (Round answers…
A: Step 1:3) Gross profit ratio:-2020:-Gross profit ratio=Gross profit / net sales…
Q: The following are selected account balances from Penske Company and Stanza Corporation as of…
A: Consolidated Net Income:Consolidated net income is the income of two or more companies or two or…
Q: Hanshaben
A: To calculate the tax consequences of these transactions to RRK Corporation, we need to consider the…
Q: The President of Leyesa Corporation was informed that production costs were 3% lower in 2023 than…
A: The objective of the question is to understand why the gross margin for 2022 was higher than that of…
Q: Farmland Company produces milk ob its farms. The entity produces 20% of the community's milk that is…
A: "Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Q: Question 5 The XYZ Partnership is being formed by three partners. Their ownership in the…
A: The objective of the question is to determine the tax year that the XYZ Partnership can elect…
Q: Kristen Lu purchased a used automobile for $13,200 at the beginning of last year and incurred the…
A: Average cost per mile$0.38 THANK YOU
Q: eBook ssignmentSession... Current Position Analysis PepsiCo, Inc. (PEP), the parent company of…
A: Step 1:a) calculation of (1) Current Ratio and (2) quick ratio The current ratio is the ratio of…
Q: On September 1, 2023, Tipton Enterprises borrowed $400,000 on a mortgage to purchase land and a…
A: Calculate the balance in the Mortgage Payable account after the October 31 payment using Excel as…
Q: aaa Ltd acquires 100% interest in YYY Ltd. On 1 July 2022 aax Ltd sells an item of plant to YYY Ltd…
A: 3. Adjust the carrying value of the plant in aaX Ltd's accounts: Dr. Accumulated Depreciation…
Q: P Corp acquired 90% of the outstanding ordinary share of B Company. On March 31, 2030, P sold…
A: Non-controlling interest is also known as a minority interest. It shows the position of ownership.…
Q: XXX Ltd acquires 100% interest in YYY Ltd. On 1 July 2022 XXX Ltd sells an item of plant to YYY Ltd…
A: The objective of the question is to provide the consolidation journal entries for the sale of an…
Q: Dorsey Company manufactures three products from a common input in a joint processing operation.…
A: Part 2: Explanation:To determine the financial advantage or disadvantage of further processing each…
Q: How often should a business prove cash? daily weekly…
A: The objective of the question is to determine the frequency at which a business should prove…
Q: Urmila
A: Step 1: Calculation of consolidated taxable income: All Taxable Income: Foreign Source IncomeU.S.…
Q: EX 9-25 (Algo) Budgeting Production and Raw-Material Purchases (LO 9-3, 9-6) GreenThumb Organic…
A: 1. Compute the company's total required production in units of finished product for the entire…
Q: None
A: Explanation: a) To interpret the confusion matrix in Figure 5.13, let's first understand the layout…
Q: Your firm has 2 deferred tax assets newly originated in 2023: One related to warranty expenses: your…
A: To prepare the 2023 income taxes entry on 12/31/2023, we need to calculate the current tax expense…
Q: None
A: 2. Now, let's calculate the variances for setup cost:a. Variance for Setup Cost using Activity-Based…
Q: Moon Manufacturing budgeted fixed overhead costs of $2.75 per unit at an anticipated production…
A: Step 1: To calculate the fixed overhead volume variance, we need to find the difference between the…
Q: James Cor. is in its first year of operations. The company has pretax income of $400,000. The…
A: Part 2: Explanation:Step 1: Calculate the tax depreciation for the equipment using the double…
Q: CASE DISCUSSION: 。 Leni and Isko are partners of Lenko Partnership which is currently liquidating.…
A: The liquidation of a partnership can be done by partners by mutual consent. Liquidation means…
Q: Dinesh
A: 1. Per Unit Mineral Cost (a):Add up the total costs associated with the minerals: purchase cost…
Q: Determine the tax liability for tax year 2021 In each of the following Instances. In each case,…
A: "Since you have asked a question with sub-parts more than three, as per guidelines, the first three…
Q: Aquatic Line Company (ALC) manufactures a variety of strong and durable ropes. The company…
A: Part 2: Explanation:Step 1: Identify Relevant Revenues and Costs:First, I listed all revenues and…
Q: Buffalo Corporation made the following cash purchases of securities during 2025, which is the first…
A: a) The journal entries to record the above three security purchases: DateParticular Debit Credit…
Q: Snackums, Incorporated, purchases wheat for use in its food manufacturing process. Snackums operates…
A: Snackums, Incorporated, operates in a highly competitive industry where increasing sales prices is…
Q: Required Information Exercise 5-44, 5-45, 5-46, 5-47, 5-48, 5-49, and 5-50 (Static) (LO 5-1, 2, 3)…
A: Store Cost Estimate for SE-18Following the provided regression equation and rounding to 2…
Q: Please help me solve ALL 5 requirements.
A: 1-a. Financial Advantage/Disadvantage of Increased Fixed Selling ExpensesIncreased Sales: 25%…
Q: Mark is a company director. His monthly salary is £8,000. During the year 2020-21, he is provided…
A: ContributionsThe amount that is reduced from the gross salary of an individual as part of TDS is…
Q: Leewin Brokerage Leewin Brokerage enters into a lease agreement with Bumble Motors to lease an…
A: Step 1: An automobile lease agreement between two entities is classified as an operating lease. The…
Step by step
Solved in 2 steps

- If xestimatexestimate is estimated with a relative error of 0.001251 and an absolute error of 0.0006, then the possible value of xx is: a. -0.480 b. -0.481 c. -0.4797 d. None e. -0.47962Find the correlation of assets A and B if the standard deviation of A is 4 and standard deviation of asset B is 2. The covariance of asset A and B is 1.8268.Which of the following is an inexact quantity?(a) the number of people in your chemistry class
- In Figure 23-29, a proton is a distance d/2 directly above the center of a square of side d. What is the magnitude of the electric flux through the square? (Hint: Think of the square as one face of a cube with edge d.)Consider the following factors. 1. (F/P,21%,34) 2. (A/G,13%,45) Find the numerical values of the factors using linear interpolation The numerical value of factor 1 is 445.79 Incorrect The numerical value of factor 2 is 7.50 7.50 CorrectThe following is a set of data from a sample of n =11items Complete parts (a) through left parenthesis c right parenthesis . X 6 36 27 54 21 39 24 9 39 39 33 Y 2 12 9 18 7 13 8 3 13 13 11 a. Compute the covariance. (Round to three decimal places as needed.) b. Compute the coefficient of correlation. (Do not round until the final answer. Then round to three decimal places as needed.) c. How strong is the relationship between X and Y? Explain. A. X and Y have no correlation. B. X and Y have a perfect positive correlation because all points fall on a straight line with a positive slope. C. X and Y have a perfect negative correlation because all points fall on a straight line with a negative slope. D. X and Y have a strong positive correlation because as X increases, Y tends to increase also.
- 2 Find the combination of inputs so that PV will equal exactly 18093.3446515882 And FV will equal exactly 57395.1490763124For a set of positively correlated data, you calculate that 84% of the variation in the dependent variable is explained by variation in the independent variable. What is the correlation coefficient(r)? (round to 3 decimal places)Use the trial and error method combined with linear interpolation to solve for the IRR of The Judas design (to one decimal place, XX.X%)
- q27- Which of the following related to the Correlation coefficient (r) are true? Select one: a. r = +1: a perfect linear relationship exists between the two variables b. r > 1: a non-linear relationship between the two variables c. r = 0: a weak linear relationship between the two variables d. r = -1: no linear relationship exists between the two variablesFind the numerical value of the factor (P/A,16.3%,15).In Elliott Wave Analysis, a full cycle is made up of how many waves? a. Impulse waves only b. 8 c. 3 d. 5



