A compound with the molecula Tormula 1202 has IR stretch at 1710 cm-1 and the 1H NMR. a) Calculate the compound's elements of unsaturation. b) What functional group does the IR indicate? c) What is the structure of the compound?
A compound with the molecula Tormula 1202 has IR stretch at 1710 cm-1 and the 1H NMR. a) Calculate the compound's elements of unsaturation. b) What functional group does the IR indicate? c) What is the structure of the compound?
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 48AP: The infrared spectrum of the compound with the mass spectrum shown below lacks any significant...
Related questions
Question
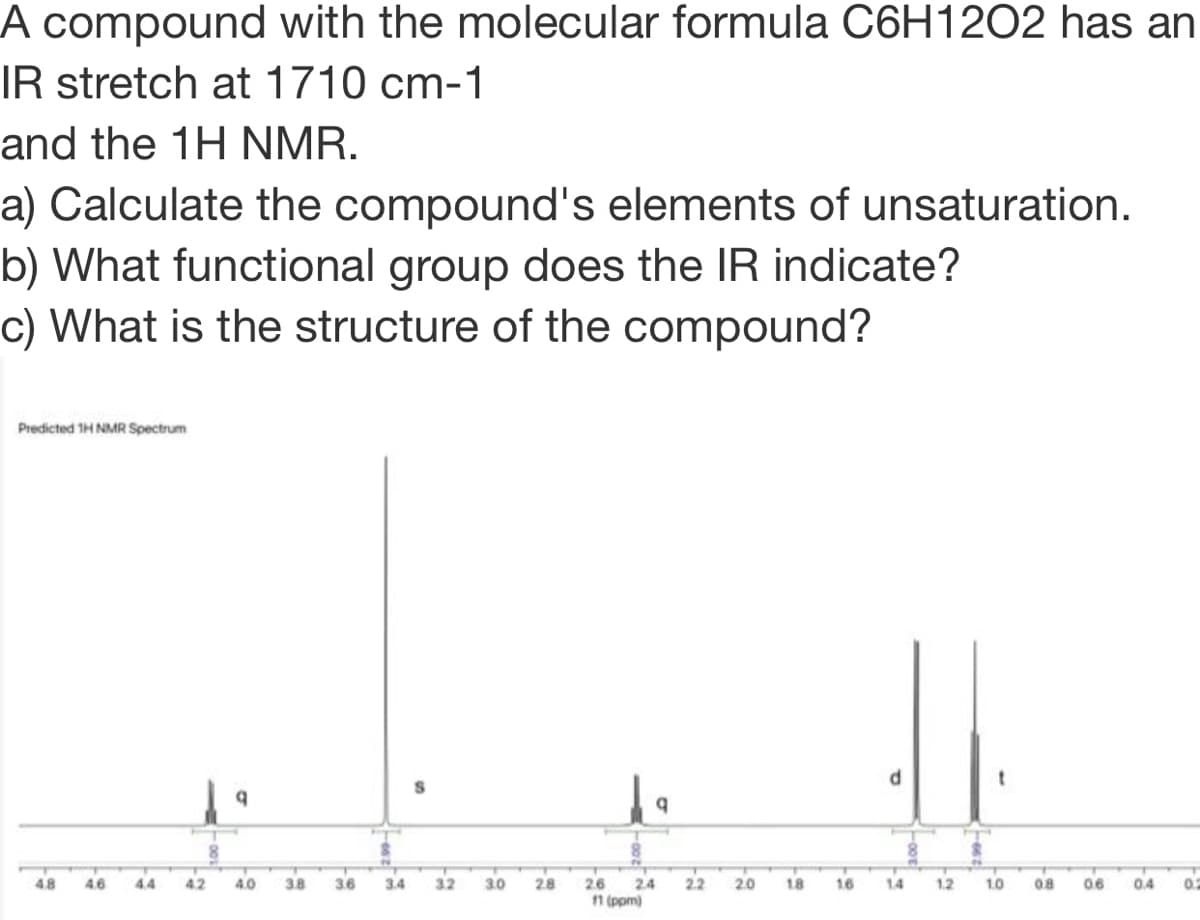
Transcribed Image Text:A compound with the molecular formula C6H12O2 has an
IR stretch at 1710 cm-1
and the 1H NMR.
a) Calculate the compound's elements of unsaturation.
b) What functional group does the IR indicate?
c) What is the structure of the compound?
Predicted 1H NMR Spectrum
d
4.8
4.6
4.4
42
4.0
38
3.6
3.4
3.2
3.0
2.8
0.4
26
2.4
1 (ppm)
2.2
2.0
18
16
1.4
1.2
1.0
08
06
02
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole