(a) Consider the following equation NO:(g) + H;O(1) - HNO:(aq) + NO(g) () Write two balanced half-equations and hence balance the above equation. (ii) Describe the redox behaviour of NO, in this reaction. (ii) State why this reaction is important industrially. A closo borane anion has the following structure (i) Write down the formula of this closo borane anion. (ii) Write down the number of skeletal electron pairs (SEP) in the anion. (iii) Determine the formula of the neutral boron hydride that can be formed by removing two of the vertices of the above borane anion and state the styx code for this neutral borane.
(a) Consider the following equation NO:(g) + H;O(1) - HNO:(aq) + NO(g) () Write two balanced half-equations and hence balance the above equation. (ii) Describe the redox behaviour of NO, in this reaction. (ii) State why this reaction is important industrially. A closo borane anion has the following structure (i) Write down the formula of this closo borane anion. (ii) Write down the number of skeletal electron pairs (SEP) in the anion. (iii) Determine the formula of the neutral boron hydride that can be formed by removing two of the vertices of the above borane anion and state the styx code for this neutral borane.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.154QP: An electrode is prepared from liquid mercury in contact with a saturated solution of mercury(I)...
Related questions
Question
100%
X
Solve question 2 b part only plz solve this now i need solve b part plz
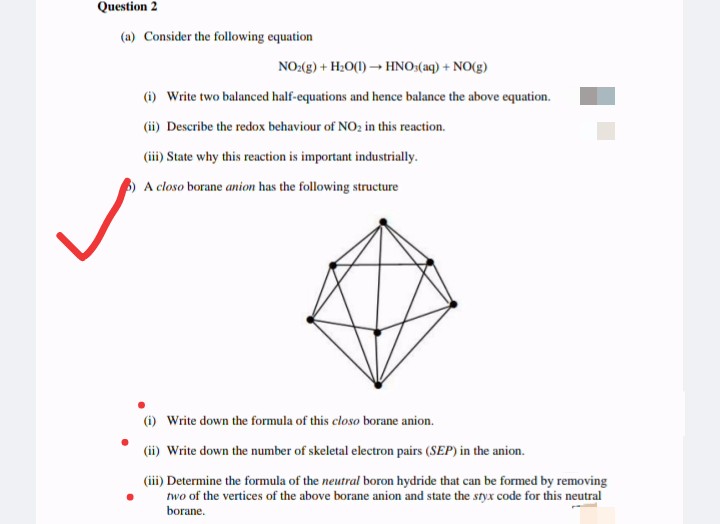
Transcribed Image Text:Question 2
(a) Consider the following equation
NO:(g) + H:0(1) → HNO:(aq) + NO(g)
(i) Write two balanced half-equations and hence balance the above equation.
(ii) Describe the redox behaviour of NO; in this reaction.
(iii) State why this reaction is important industrially.
A closo borane anion has the following structure
(i) Write down the formula of this closo borane anion.
(ii) Write down the number of skeletal electron pairs (SEP) in the anion.
(iii) Determine the formula of the neutral boron hydride that can be formed by removing
two of the vertices of the above borane anion and state the styx code for this neutral
borane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning