A cup contains 187 g of coffee at 97.6 °C. Suppose 56.2 g of ice is added to the coffee. What is the temperature of the coffee after all of the ice melts? The enthalpy of fusion of water can be found in this table. Assume the specific heat capacity of the coffee is the same as water.
A cup contains 187 g of coffee at 97.6 °C. Suppose 56.2 g of ice is added to the coffee. What is the temperature of the coffee after all of the ice melts? The enthalpy of fusion of water can be found in this table. Assume the specific heat capacity of the coffee is the same as water.
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 2P: Convert the following to equivalent temperatures on the Celsius and Kelvin scales: (a) the normal...
Related questions
Question
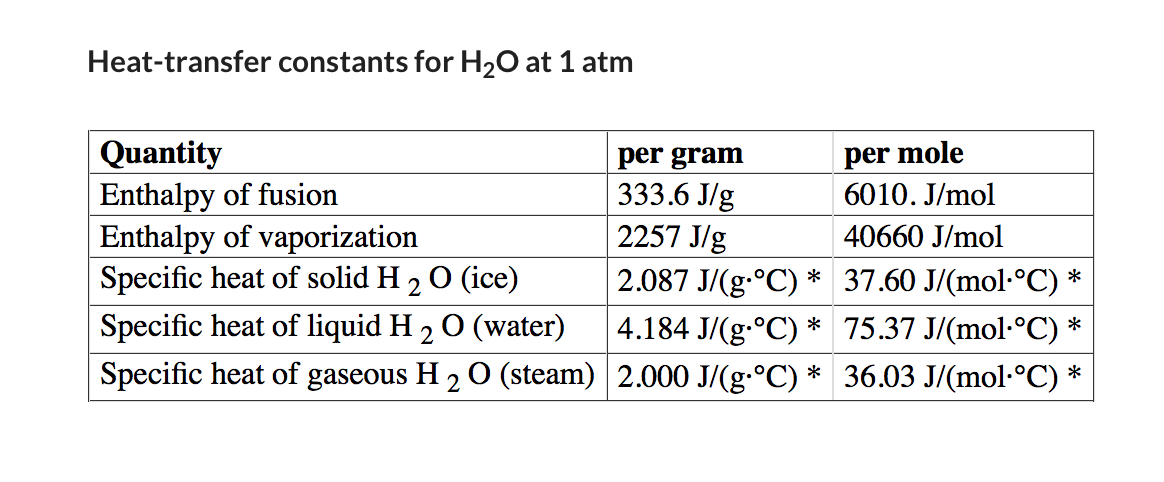
Transcribed Image Text:Heat-transfer constants for H20 at 1 atm
Quantity
Enthalpy of fusion
Enthalpy of vaporization
Specific heat of solid H 20 (ice)
per gram
| 333.6 J/g
2257 J/g
per mole
6010. J/mol
40660 J/mol
2.087 J/(g.°C) * 37.60 J/(mol-°C) *
|4.184 J/(g.°C) * 75.37 J/(mol·°C) *
Specific heat of gaseous H 2 0 (steam) 2.000 J/(g-°C) * 36.03 J/(mol-°C) *
Specific heat of liquid H 2 O (water)

Transcribed Image Text:A cup contains 187 g of coffee at 97.6 °C. Suppose 56.2 g of ice is added to the coffee. What is the temperature of the coffee
after all of the ice melts? The enthalpy of fusion of water can be found in this table. Assume the specific heat capacity of the
coffee is the same as water.
Tinal
°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning