A cylindrical metal canister is filled with pressurized diatomic oxygen gas, O2. e circular base of the cylinder has radius of 0,060 m, and the height of the cylinder is 0.300 m. e gas pressure is 5.78 x 10 Pa. The canister and oxygen gas are at room temperature, 23.5° C. a. What is the volume of the cylindrical canister, in m' ? b. How many moles of oxygen gas are in the cylindrical canister ? c. What is the total average kinetic energy per molecule, in this gas (all modes) ?
A cylindrical metal canister is filled with pressurized diatomic oxygen gas, O2. e circular base of the cylinder has radius of 0,060 m, and the height of the cylinder is 0.300 m. e gas pressure is 5.78 x 10 Pa. The canister and oxygen gas are at room temperature, 23.5° C. a. What is the volume of the cylindrical canister, in m' ? b. How many moles of oxygen gas are in the cylindrical canister ? c. What is the total average kinetic energy per molecule, in this gas (all modes) ?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 76QAP: The Lamborghini Aventador engine has a 12-cylinder engine in which each cylinder has a volume of 542...
Related questions
Question
I’m stuck on this home work problem please help
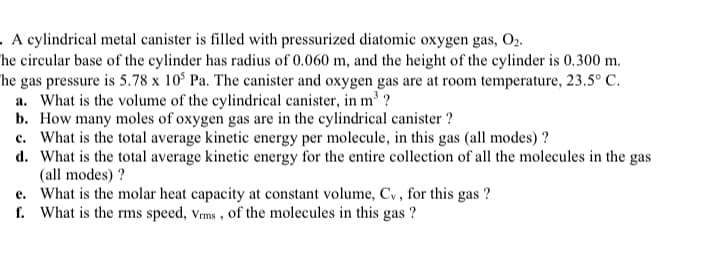
Transcribed Image Text:. A cylindrical metal canister is filled with pressurized diatomic oxygen gas, O2.
he circular base of the cylinder has radius of 0.060 m, and the height of the cylinder is 0.300 m.
he gas pressure is 5.78 x 10° Pa. The canister and oxygen gas are at room temperature, 23.5° C.
a. What is the volume of the cylindrical canister, in m' ?
b. How many moles of oxygen gas are in the cylindrical canister ?
c. What is the total average kinetic energy per molecule, in this gas (all modes) ?
d. What is the total average kinetic energy for the entire collection of all the molecules in the gas
(all modes) ?
e. What is the molar heat capacity at constant volume, Cy, for this gas ?
f. What is the rms speed, Vms , of the molecules in this gas ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning