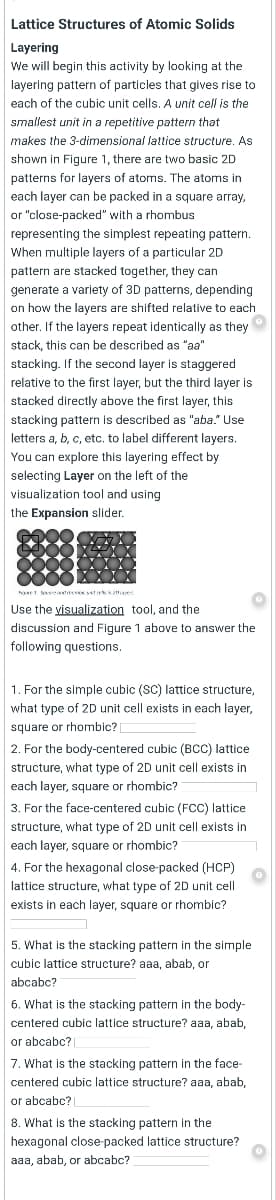
Lattice Structures of Atomic Solids Layering We will begin this activity by looking at the layering pattern of particles that gives rise to each of the cubic unit cells. A unit cell is the smallest unit in a repetitive pattern that makes the 3-dimensional lattice structure. As shown in Figure 1, there are two basic 2D patterns for layers of atoms. The atoms in each layer can be packed in a square array, or "close-packed" with a rhombus representing the simplest repeating pattern. When multiple layers of a particular 2D pattern are stacked together, they can generate a variety of 3D patterns, depending on how the layers are shifted relative to each other. If the layers repeat identically as they stack, this can be described as "aa" stacking. If the second layer is staggered relative to the first layer, but the third layer is
Lattice Structures of Atomic Solids Layering We will begin this activity by looking at the layering pattern of particles that gives rise to each of the cubic unit cells. A unit cell is the smallest unit in a repetitive pattern that makes the 3-dimensional lattice structure. As shown in Figure 1, there are two basic 2D patterns for layers of atoms. The atoms in each layer can be packed in a square array, or "close-packed" with a rhombus representing the simplest repeating pattern. When multiple layers of a particular 2D pattern are stacked together, they can generate a variety of 3D patterns, depending on how the layers are shifted relative to each other. If the layers repeat identically as they stack, this can be described as "aa" stacking. If the second layer is staggered relative to the first layer, but the third layer is
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.4E
Related questions
Question
Transcribed Image Text:Lattice Structures of Atomic Solids
Layering
We will begin this activity by looking at the
layering pattern of particles that gives rise to
each of the cubic unit cells. A unit cell is the
smallest unit in a repetitive pattern that
makes the 3-dimensional lattice structure. As
shown in Figure 1, there are two basic 2D
patterns for layers of atoms. The atoms in
each layer can be packed in a square array,
or "close-packed" with a rhombus
representing the simplest repeating pattern.
When multiple layers of a particular 2D
pattern are stacked together, they can
generate a variety of 3D patterns, depending
on how the layers are shifted relative to each
other. If the layers repeat identically as they
stack, this can be described as "aa"
stacking. If the second layer is staggered
relative to the first layer, but the third layer is
stacked directly above the first layer, this
stacking pattern is described as "aba." Use
letters a, b, c, etc, to label different layers.
You can explore this layering effect by
selecting Layer on the left of the
visualization tool and using
the Expansion slider.
ae sseatace tesk naes
Use the visualization tool, and the
discussion and Figure 1 above to answer the
following questions.
1. For the simple cubic (SC) lattice structure,
what type of 2D unit cell exists in each layer,
square or rhombic?
2. For the body-centered cubic (BCC) lattice
structure, what type of 2D unit cell exists in
each layer, square or rhombic?
3. For the face-centered cubic (FCC) lattice
structure, what type of 2D unit cell exists in
each layer, square or rhombic?
4. For the hexagonal close-packed (HCP)
lattice structure, what type of 2D unit cell
exists in each layer, square or rhombic?
5. What is the stacking pattern in the simple
cubic lattice structure? aaa, abab, or
abcabc?
6. What is the stacking pattern in the body-
centered cubic lattice structure? aaa, abab,
or abcabc? |
7. What is the stacking pattern in the face-
centered cubic lattice structure? aaa, abab,
or abcabc? |
8. What is the stacking pattern in the
hexagonal close-packed lattice structure?
aaa, abab, or abcabc?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,