a) Determine either the type of the following reaction is addition, substitution or elimination reaction. + Cl2 FeCl b) Construct a complete structural formula by adding hydrogen to compound A and B. Then write the molecular formula of each compound. C-c=c-C С —с—с—с Compound A Compound B
a) Determine either the type of the following reaction is addition, substitution or elimination reaction. + Cl2 FeCl b) Construct a complete structural formula by adding hydrogen to compound A and B. Then write the molecular formula of each compound. C-c=c-C С —с—с—с Compound A Compound B
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 123QRT
Related questions
Question
How to do these questions?
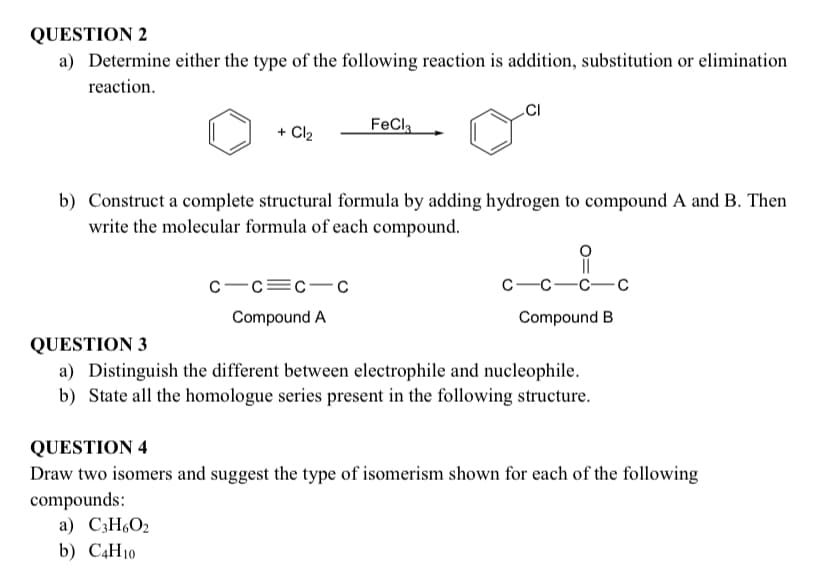
Transcribed Image Text:QUESTION 2
a) Determine either the type of the following reaction is addition, substitution or elimination
reaction.
CI
+ Cl2
FeCla
b) Construct a complete structural formula by adding hydrogen to compound A and B. Then
write the molecular formula of each compound.
c-c=c-c
с —с—с—с
Compound A
Compound B
QUESTION 3
a) Distinguish the different between electrophile and nucleophile.
b) State all the homologue series present in the following structure.
QUESTION 4
Draw two isomers and suggest the type of isomerism shown for each of the following
compounds:
a) C3H6O2
b) C4H10
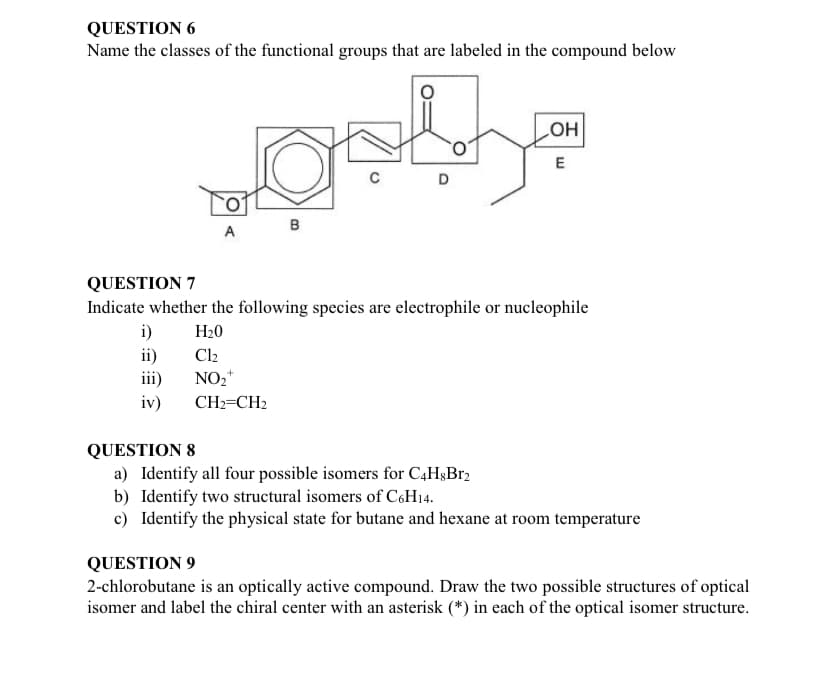
Transcribed Image Text:QUESTION 6
Name the classes of the functional groups that are labeled in the compound below
HO
E
D
в
A
QUESTION 7
Indicate whether the following species are electrophile or nucleophile
i)
H20
Cl2
ii)
iii)
NO2*
iv)
CH2=CH2
QUESTION 8
a) Identify all four possible isomers for C4H&Br2
b) Identify two structural isomers of C6H14.
c) Identify the physical state for butane and hexane at room temperature
QUESTION 9
2-chlorobutane is an optically active compound. Draw the two possible structures of optical
isomer and label the chiral center with an asterisk (*) in each of the optical isomer structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning