A different way to produce ethanol (C2H60, M.M. = 46.08 g/mol) is through fermentation of sugar (C6H1206, M.M. = 180.18 g/mol) Balance the following chemical equation: + C6H12O6(aq) → : C2H6O(aq) + CO2(g) Using your balanced chemical equation from the previous question, determine what mass of C6H1206 (in grams) is needed to make 10.0 g of ethanol. Make sure to report your answer to the correct number of significant figures and with the correct units. Answer: Using your answer from the previous question, determine the percent yield of the reaction if you actually produce 7.50 g of ethanol. Make sure to report your answer to the correct number of significant figures and with the correct units. Answer:
A different way to produce ethanol (C2H60, M.M. = 46.08 g/mol) is through fermentation of sugar (C6H1206, M.M. = 180.18 g/mol) Balance the following chemical equation: + C6H12O6(aq) → : C2H6O(aq) + CO2(g) Using your balanced chemical equation from the previous question, determine what mass of C6H1206 (in grams) is needed to make 10.0 g of ethanol. Make sure to report your answer to the correct number of significant figures and with the correct units. Answer: Using your answer from the previous question, determine the percent yield of the reaction if you actually produce 7.50 g of ethanol. Make sure to report your answer to the correct number of significant figures and with the correct units. Answer:
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.20QP: Propane, C3H8, is the fuel of choice in a gas barbecue. When burning, the balanced equation is...
Related questions
Question
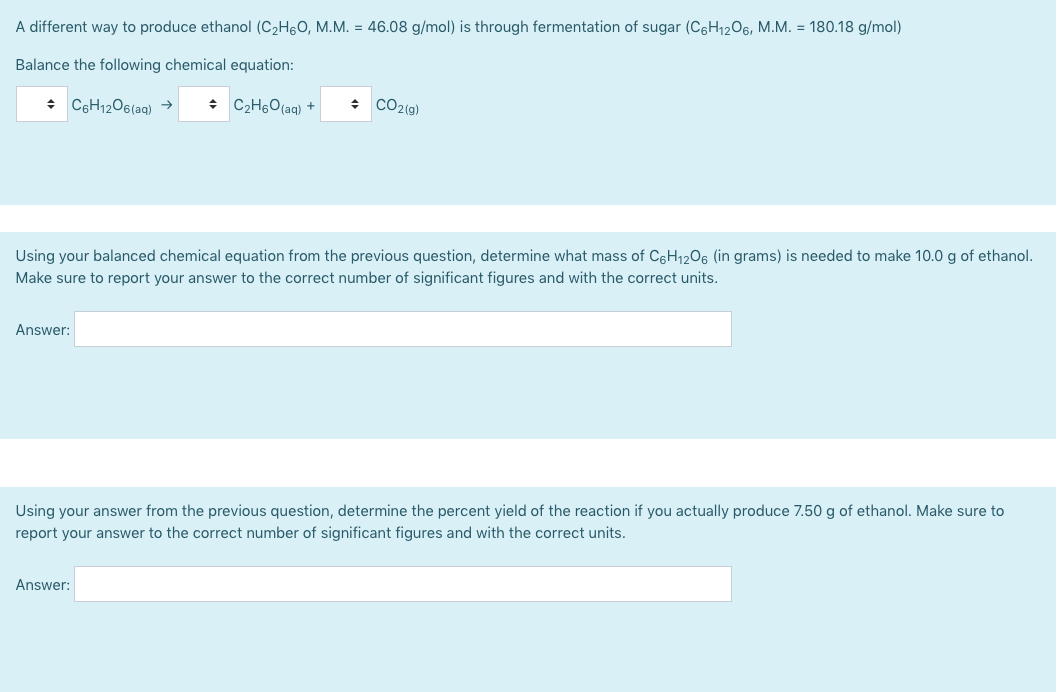
Transcribed Image Text:A different way to produce ethanol (C2H60, M.M. = 46.08 g/mol) is through fermentation of sugar (C6H1206, M.M. = 180.18 g/mol)
Balance the following chemical equation:
+ C6H12O6(aq) →
: C2H6O(aq) +
CO2(g)
Using your balanced chemical equation from the previous question, determine what mass of C6H1206 (in grams) is needed to make 10.0 g of ethanol.
Make sure to report your answer to the correct number of significant figures and with the correct units.
Answer:
Using your answer from the previous question, determine the percent yield of the reaction if you actually produce 7.50 g of ethanol. Make sure to
report your answer to the correct number of significant figures and with the correct units.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning