A diver at the bottom of a lake, where the water temperature is 4.0 °C, observes a bubble of gas travel up to the surface of the lake. For this lake, at the surface the pressure is 1.0 atmosphere and the water temperature is 27.0 °C. The ratio of the bubble volume at the surface to the bubble volume at the bottom of the lake is 5.42. Assume the bubble behaves as an ideal gas. (a) Calculate the pressure, in Pascals, of the gas in the bubble at the bottom of the lake. Give your answer in scientific notation and specified to an appropriate number of significant figures, in the empty box below. pressure of gas in bubble at bottom of lake = Р₁ = Pa (b) The bubble can be assumed to be approximately spherical and contains 4.0 × 1017 molecules of gas. Calculate the radius of the bubble, in mm, at the bottom of the lake. Give your answer by entering a number, specified to an appropriate number of significant figures, in the empty box below. radius of bubble at bottom of lake = mm
A diver at the bottom of a lake, where the water temperature is 4.0 °C, observes a bubble of gas travel up to the surface of the lake. For this lake, at the surface the pressure is 1.0 atmosphere and the water temperature is 27.0 °C. The ratio of the bubble volume at the surface to the bubble volume at the bottom of the lake is 5.42. Assume the bubble behaves as an ideal gas. (a) Calculate the pressure, in Pascals, of the gas in the bubble at the bottom of the lake. Give your answer in scientific notation and specified to an appropriate number of significant figures, in the empty box below. pressure of gas in bubble at bottom of lake = Р₁ = Pa (b) The bubble can be assumed to be approximately spherical and contains 4.0 × 1017 molecules of gas. Calculate the radius of the bubble, in mm, at the bottom of the lake. Give your answer by entering a number, specified to an appropriate number of significant figures, in the empty box below. radius of bubble at bottom of lake = mm
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 50P
Related questions
Question
100%
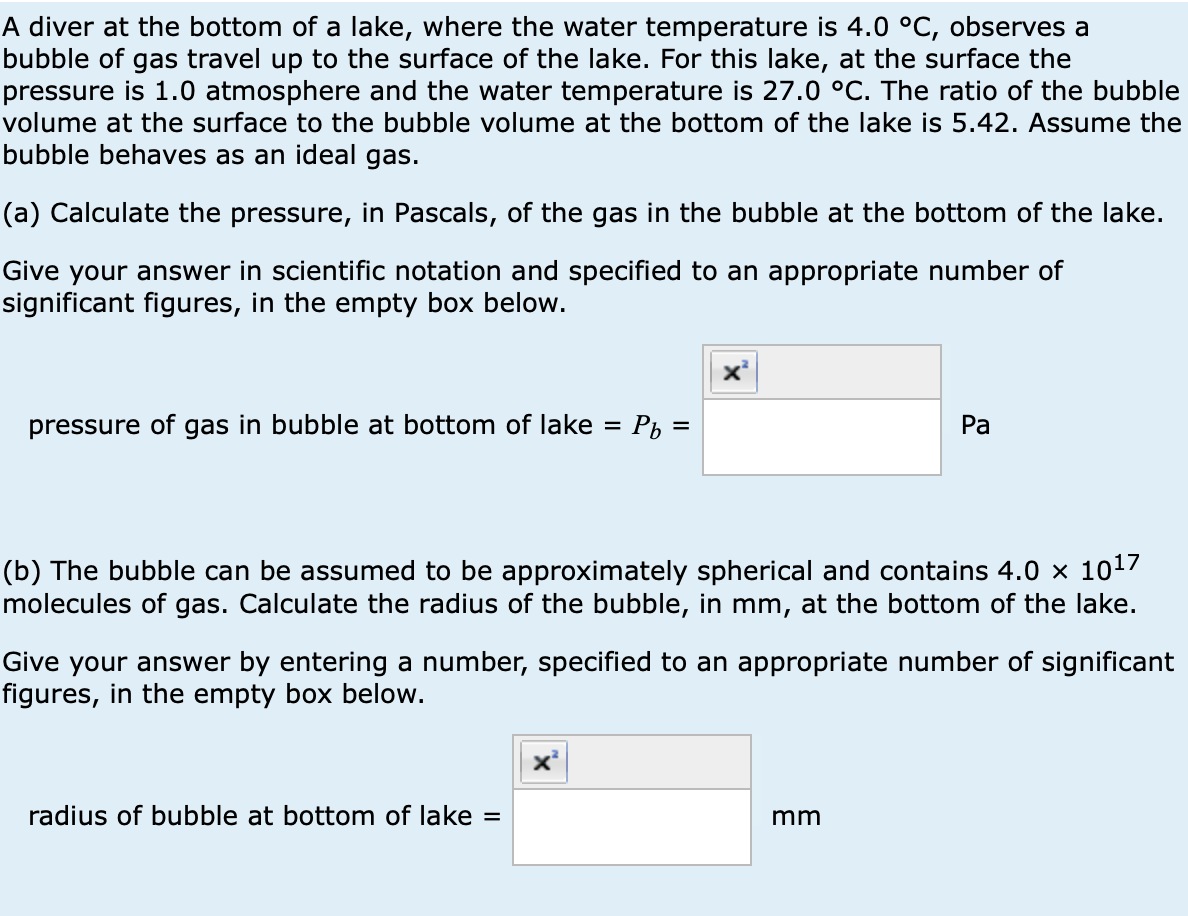
Transcribed Image Text:A diver at the bottom of a lake, where the water temperature is 4.0 °C, observes a
bubble of gas travel up to the surface of the lake. For this lake, at the surface the
pressure is 1.0 atmosphere and the water temperature is 27.0 °C. The ratio of the bubble
volume at the surface to the bubble volume at the bottom of the lake is 5.42. Assume the
bubble behaves as an ideal gas.
(a) Calculate the pressure, in Pascals, of the gas in the bubble at the bottom of the lake.
Give your answer in scientific notation and specified to an appropriate number of
significant figures, in the empty box below.
pressure of gas in bubble at bottom of lake = Р₁ =
Pa
(b) The bubble can be assumed to be approximately spherical and contains 4.0 × 1017
molecules of gas. Calculate the radius of the bubble, in mm, at the bottom of the lake.
Give your answer by entering a number, specified to an appropriate number of significant
figures, in the empty box below.
radius of bubble at bottom of lake =
mm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 2 images

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning