A few different types of electromagnetic radiation are listed in the table below. Complete the table by ordering these types of radiation by increasing frequency, wavelength, and energy per photon. For example, select "1" in the second column for the type of radiation with the lowest frequency, 2" for the type of radiation with the next higher frequency, and so forth. type of radiation order of frequency order of wavelength order of energy radio waves (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ gamma rays (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ microwaves (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ green light (Choose one) ▼ (Choose one) ▼ (Choose one) ▼
A few different types of electromagnetic radiation are listed in the table below. Complete the table by ordering these types of radiation by increasing frequency, wavelength, and energy per photon. For example, select "1" in the second column for the type of radiation with the lowest frequency, 2" for the type of radiation with the next higher frequency, and so forth. type of radiation order of frequency order of wavelength order of energy radio waves (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ gamma rays (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ microwaves (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ green light (Choose one) ▼ (Choose one) ▼ (Choose one) ▼
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 45AP: Suppose an atom in an excited state can return to the ground state in two steps. It first falls to...
Related questions
Question
100%
1 being the shortest/lowest and 4 being the longest/highest
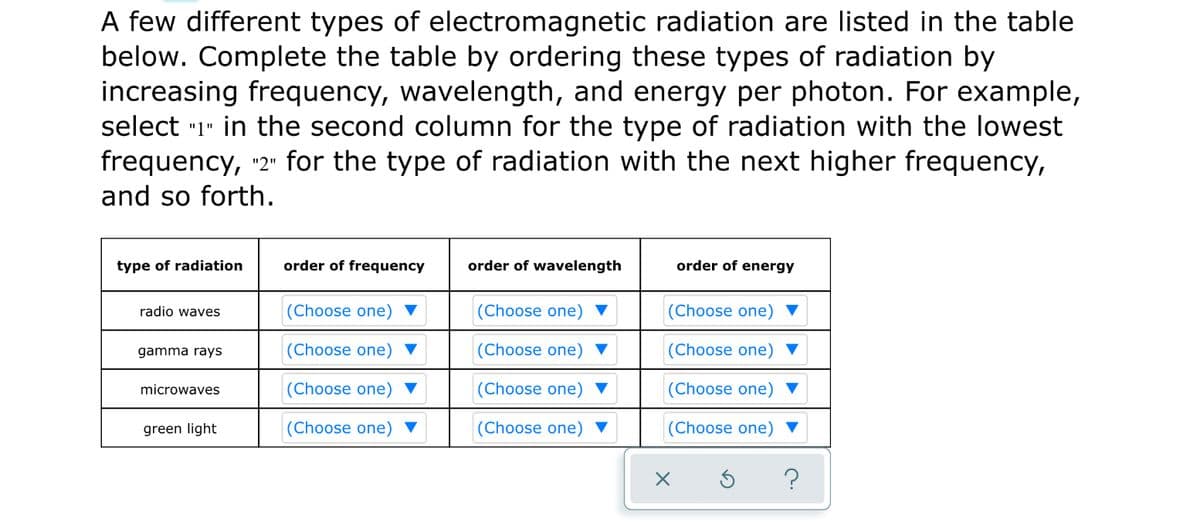
Transcribed Image Text:A few different types of electromagnetic radiation are listed in the table
below. Complete the table by ordering these types of radiation by
increasing frequency, wavelength, and energy per photon. For example,
select "1" in the second column for the type of radiation with the lowest
frequency, "2" for the type of radiation with the next higher frequency,
and so forth.
type of radiation
order of frequency
order of wavelength
order of energy
radio waves
(Choose one)
(Choose one)
(Choose one)
gamma rays
(Choose one) ▼
(Choose one) ▼
(Choose one)
microwaves
(Choose one) ▼
(Choose one) ▼
(Choose one)
green light
(Choose one) ▼
(Choose one) ▼
(Choose one) ▼
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning