A galvanic cell is made with a metal "A" electrode placed in a 1.0 M solution of metal "A" sulfate, ASO4, making up one half-cell and metal "B" electrode placed in a 1.0 M solution of metal "B" sulfate, BSO4, making up the other half-cell. The resulting galavanic cell is pictured below. Fill in the following statements as related to the galvanic cell pictured below. (Oxidation or Reduction) ______ occurs in the half-cell with the "A" electrode. The (A or B) electrode will increase in mass. The electrons will flow toward the half cell with the (A or B) electrode. The reaction occurring with the lower reduction potential is in the half-cell with the (A or B) electrode. ~~~~~ Referring to the diagram of the galvanic cell in the previous question, what is the half-reaction that occurs in the cell on the right? A) B(s) + 2e−→ B2+(aq) B) B2+(aq) → B(s) + 2e− C) B(s) → B2+(aq) + 2e− D) B2+(aq) + 2e−→ B(s)
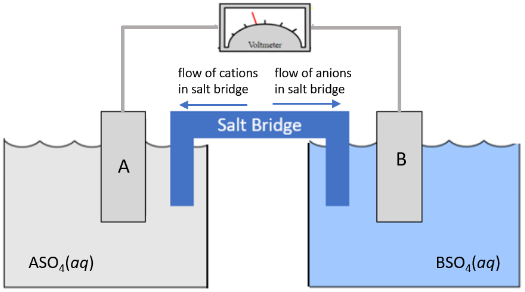
A galvanic cell is made with a metal "A" electrode placed in a 1.0 M solution of metal "A" sulfate, ASO4, making up one half-cell and metal "B" electrode placed in a 1.0 M solution of metal "B" sulfate, BSO4, making up the other half-cell. The resulting galavanic cell is pictured below.
Fill in the following statements as related to the galvanic cell pictured below.
(Oxidation or Reduction) ______ occurs in the half-cell with the "A" electrode.
The (A or B) electrode will increase in mass.
The electrons will flow toward the half cell with the (A or B) electrode.
The reaction occurring with the lower reduction potential is in the half-cell with the (A or B) electrode.
~~~~~
Referring to the diagram of the galvanic cell in the previous question, what is the half-reaction that occurs in the cell on the right?
A) B(s) + 2e−→ B2+(aq)
B) B2+(aq) → B(s) + 2e−
C) B(s) → B2+(aq) + 2e−
D) B2+(aq) + 2e−→ B(s)
Trending now
This is a popular solution!
Step by step
Solved in 6 steps







