A gas in a cylinder expands from a volume of 0.100 m³ to 0.330 m3 . Heat flows into the gas just rapidly enough to keep the pressure constant at 1.90x105 Pa during the expansion. The total heat added is 1.20x105 J. For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Thermodynamics of boiling water. Part A Find the work done by the gas. Express your answer in joules. ΑΣφ ? W J Submit Request Answer Part B
A gas in a cylinder expands from a volume of 0.100 m³ to 0.330 m3 . Heat flows into the gas just rapidly enough to keep the pressure constant at 1.90x105 Pa during the expansion. The total heat added is 1.20x105 J. For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Thermodynamics of boiling water. Part A Find the work done by the gas. Express your answer in joules. ΑΣφ ? W J Submit Request Answer Part B
Physics for Scientists and Engineers with Modern Physics
10th Edition
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 34AP: In a cylinder, a sample of an ideal gas with number of moles n undergoes an adiabatic process. (a)...
Related questions
Question
Transcribed Image Text:12 of 13
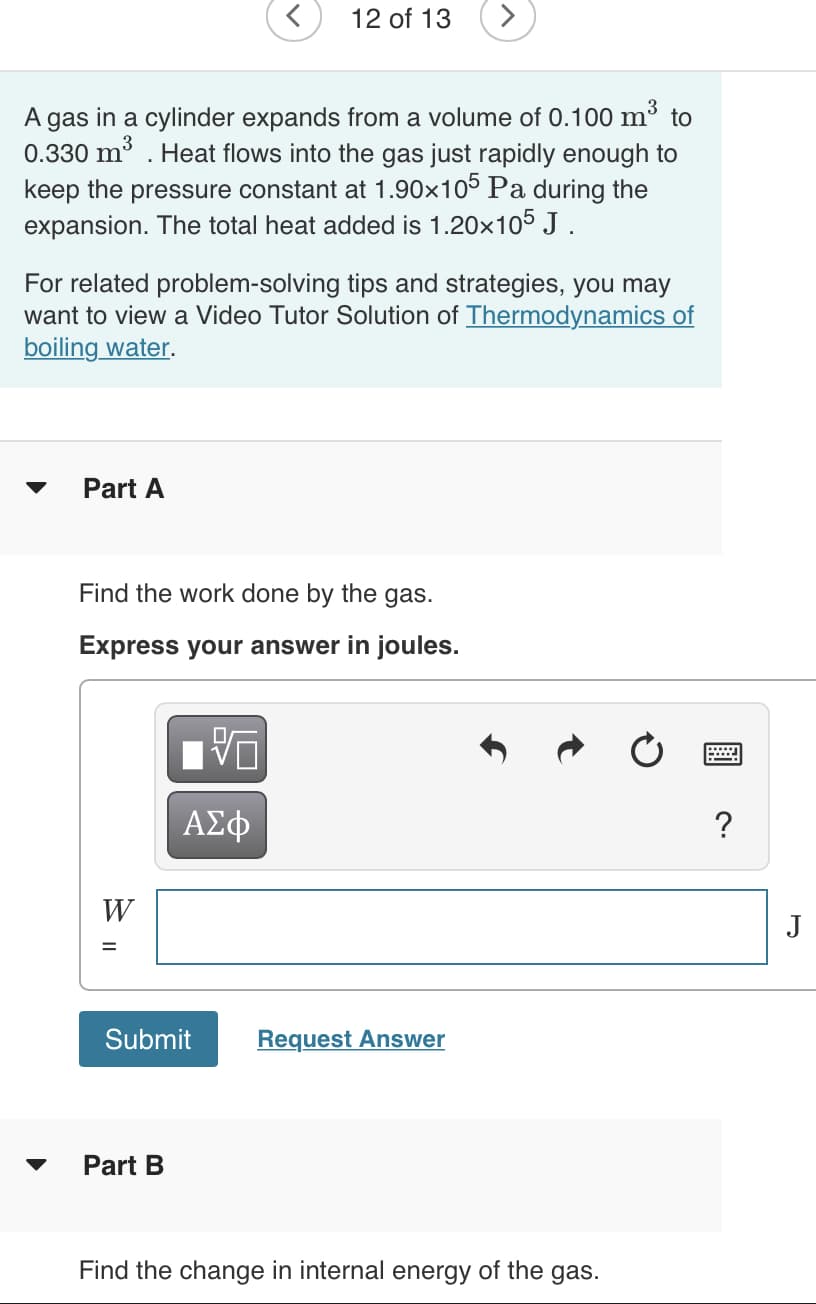
A gas in a cylinder expands from a volume of 0.100 m³ to
0.330 m . Heat flows into the gas just rapidly enough to
keep the pressure constant at 1.90x105 Pa during the
expansion. The total heat added is 1.20x105 J .
For related problem-solving tips and strategies, you may
want to view a Video Tutor Solution of Thermodynamics of
boiling water.
Part A
Find the work done by the gas.
Express your answer in joules.
?
W
J
Submit
Request Answer
Part B
Find the change in internal energy of the gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning