A gas with a volume of 640 mL at a pressure of 2.91 atm is allowed to expand to a volume of 3.58 L. What is the pressure in the container if the temperature remains constant? Be sure to enter a unit with your answer.
A gas with a volume of 640 mL at a pressure of 2.91 atm is allowed to expand to a volume of 3.58 L. What is the pressure in the container if the temperature remains constant? Be sure to enter a unit with your answer.
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 81AP: One process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of...
Related questions
Question
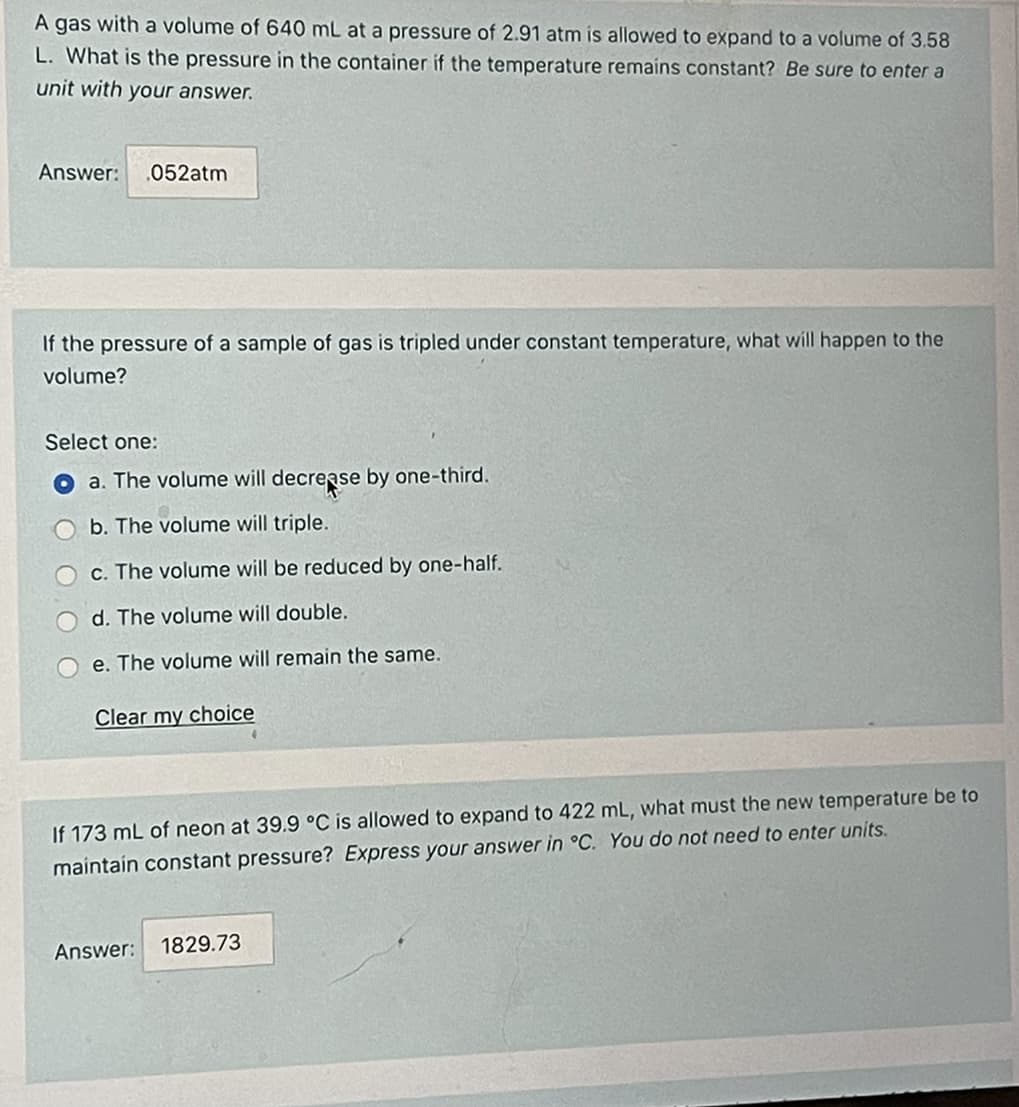
Transcribed Image Text:A gas with a volume of 640 mL at a pressure of 2.91 atm is allowed to expand to a volume of 3.58
L. What is the pressure in the container if the temperature remains constant? Be sure to enter a
unit with your answer.
Answer:
.052atm
If the pressure of a sample of gas is tripled under constant temperature, what will happen to the
volume?
Select one:
a. The volume will decrease by one-third.
b. The volume will triple.
c. The volume will be reduced by one-half.
d. The volume will double.
e. The volume will remain the same.
Clear my choice
If 173 mL of neon at 39.9 °C is allowed to expand to 422 mL, what must the new temperature be to
maintain constant pressure? Express your answer in °C. You do not need to enter units.
Answer:
1829.73
Expert Solution
Step 1
Since there are multiple questions being posted, we are answering the first question.
As the temperature remains constant, the Boyle’s law is given by
Substitute for , for , and for in the above equation.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning