A gasoline truck engine takes in 10,000 J of heat and delivers 2,000 J of mechanical work per cycle. Describe the entropy as heat flows in the system
A gasoline truck engine takes in 10,000 J of heat and delivers 2,000 J of mechanical work per cycle. Describe the entropy as heat flows in the system
Chapter4: The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 61P: The Carnot cycle is represented by the temperature-entropy diagram shown below. (a) How much heat is...
Related questions
Question
100%
A gasoline truck engine takes in 10,000 J of heat and delivers 2,000 J of mechanical work per cycle. Describe the entropy as heat flows in the system.
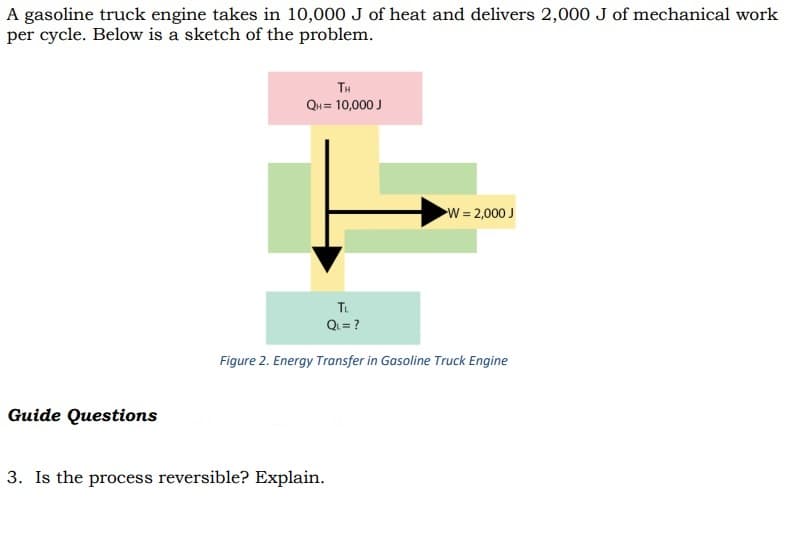
Transcribed Image Text:A gasoline truck engine takes in 10,000 J of heat and delivers 2,000 J of mechanical work
per cycle. Below is a sketch of the problem.
TH
QH = 10,000 J
W= 2,000 J
Tu
Q= ?
Figure 2. Energy Transfer in Gasoline Truck Engine
Guide Questions
3. Is the process reversible? Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

