A generic salt, AB,, has a molar mass of 295 g/mol and a solubility of 1.00 g/L at 25 °C. AB,(s)= A (aq) + 3 B (aq) What is the K of this salt at 25 C? Ksp %3D TOOLS
A generic salt, AB,, has a molar mass of 295 g/mol and a solubility of 1.00 g/L at 25 °C. AB,(s)= A (aq) + 3 B (aq) What is the K of this salt at 25 C? Ksp %3D TOOLS
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 90QAP: Consider a metal ion A2+ and its nitrate salt, In an experiment, 35.00 mL of a 0.217 M solution of...
Related questions
Question
100%
need both parts clearly and detail don't copy I will downvote
Answer only if u knows
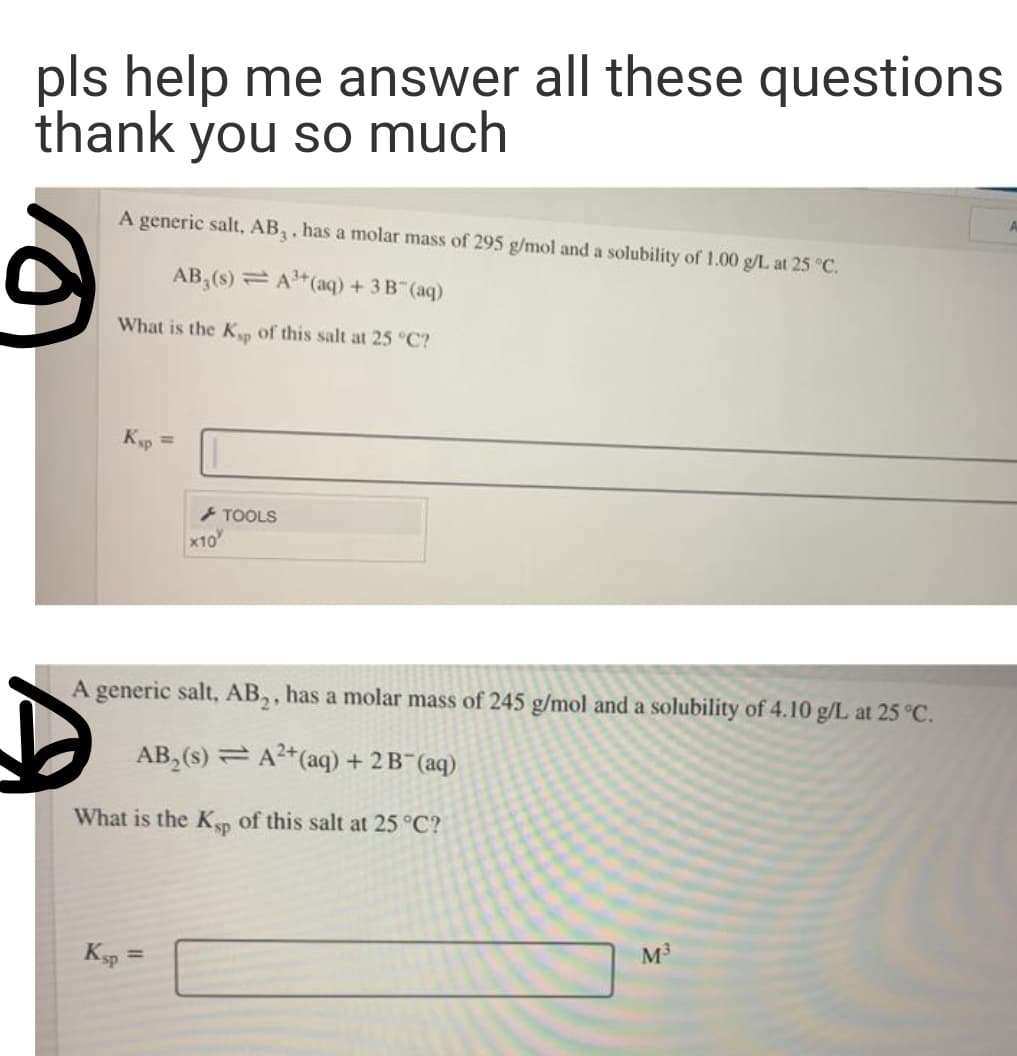
Transcribed Image Text:pls help me answer all these questions
thank you so much
A generic salt, AB,, has a molar mass of 295 g/mol and a solubility of 1.00 g/L at 25 °C.
AB,(s) = A"(aq) +3B (aq)
What is the K of this salt at 25 °C?
Kp =
TOOLS
x10
A generic salt, AB,, has a molar mass of 245 g/mol and a solubility of 4.10 g/L at 25 °C.
AB, (s) = A2*(aq) + 2 B (aq)
What is the Kp of this salt at 25 °C?
Ksp
M3
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning