AgCI(s) 2 Ag* (aq) + CIF (aq) Ksp = 4.0 x 10-0 Ag* (aq) + 2 CI (aq) 2 [AgCl] (aq) K2 = 1.8 x 105 AgCl(s) + CI (aq) 2 [AgCl,] (aq) K3 = ? () The student finds that AgCl is more soluble in NaCl(ag) than in water because of the series of reactions above. Determine the value of K3 for the overall reaction.
AgCI(s) 2 Ag* (aq) + CIF (aq) Ksp = 4.0 x 10-0 Ag* (aq) + 2 CI (aq) 2 [AgCl] (aq) K2 = 1.8 x 105 AgCl(s) + CI (aq) 2 [AgCl,] (aq) K3 = ? () The student finds that AgCl is more soluble in NaCl(ag) than in water because of the series of reactions above. Determine the value of K3 for the overall reaction.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.67QE
Related questions
Question
![AgCl(s) 2 Ag+ (aq) + CI- (aq) Ken = 4.0 x 10-10
ds.
Ag* (aq) + 2 CIF (aq) 2 [AgCl,] (aq)
K2 = 1.8 x 105
AgCl(s) + CIF (aq) [AgCl,]¯ (aq)
K3 = ?
(i) The student finds that AgCl is more soluble in NaCI(aq) than in water because of the series of reactions above. Determine the value of K3 for the overall reaction.
В I U
x2
X2 5
Ω
II](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fa60bf688-fc7c-4a1b-9f84-c733c16679e8%2F568c2b7c-f60a-4002-bf1e-5ccc431119a1%2Fmbnjvyo_processed.jpeg&w=3840&q=75)
Transcribed Image Text:AgCl(s) 2 Ag+ (aq) + CI- (aq) Ken = 4.0 x 10-10
ds.
Ag* (aq) + 2 CIF (aq) 2 [AgCl,] (aq)
K2 = 1.8 x 105
AgCl(s) + CIF (aq) [AgCl,]¯ (aq)
K3 = ?
(i) The student finds that AgCl is more soluble in NaCI(aq) than in water because of the series of reactions above. Determine the value of K3 for the overall reaction.
В I U
x2
X2 5
Ω
II
Transcribed Image Text:2 of 2
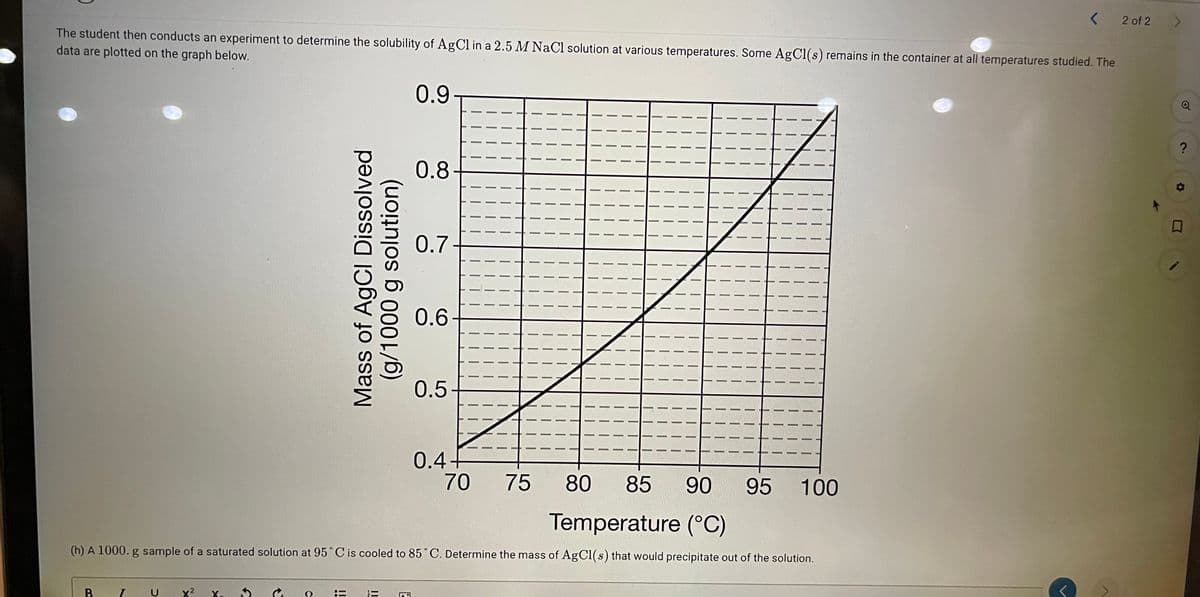
The student then conducts an experiment to determine the solubility of AgCl in a 2.5 M NaCl solution at various temperatures. Some AgCl(s) remains in the container at all temperatures studied. The
data are plotted on the graph below.
0.9
0.8
0.7
0.6
0.5-
0.4+
70
75
80
85
90
95 100
Temperature (°C)
(h) A 1000. g sample of a saturated solution at 95° C is cooled to 85°C. Determine the mass of AgCl(s) that would precipitate out of the solution.
B
U
x2
X.
Mass of AgCl Dissolved
solution)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning