A generic solid, X, has a molar mass of 69.9 g/mol. In a constant-pressure calorimeter, 47.9 g of X is dissolved in 219 g of water at 23.00 °C. X(s) → X(aq) The temperature of the resulting solution rises to 24.30 °C. Assume the solution has the same specific heat as water, 4.184 J/(g.°C), and that there is negligible heat loss to the surroundings. How much heat was absorbed by the solution? q = kJ What is the enthalpy of the AHrxn = kJ/mol
A generic solid, X, has a molar mass of 69.9 g/mol. In a constant-pressure calorimeter, 47.9 g of X is dissolved in 219 g of water at 23.00 °C. X(s) → X(aq) The temperature of the resulting solution rises to 24.30 °C. Assume the solution has the same specific heat as water, 4.184 J/(g.°C), and that there is negligible heat loss to the surroundings. How much heat was absorbed by the solution? q = kJ What is the enthalpy of the AHrxn = kJ/mol
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 104AE
Related questions
Question
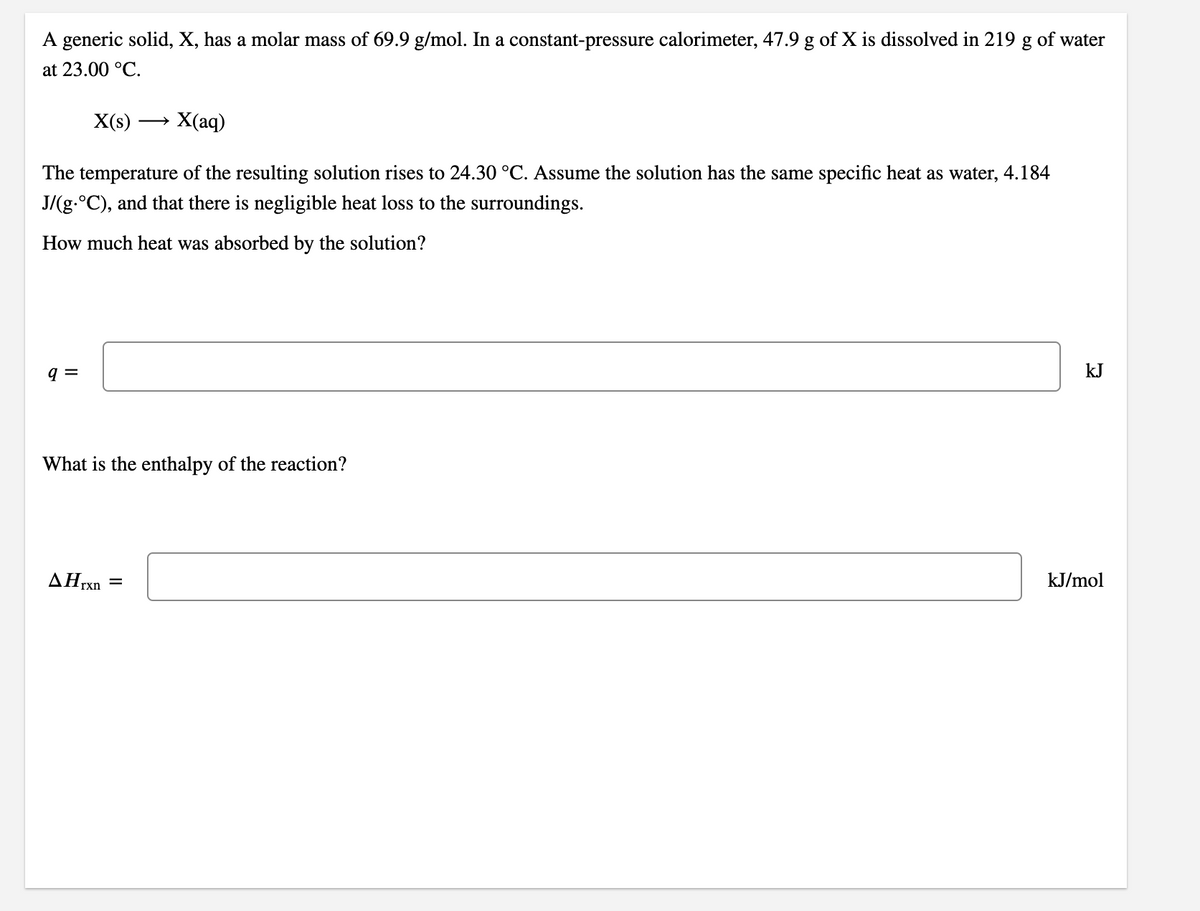
Transcribed Image Text:A generic solid, X, has a molar mass of 69.9 g/mol. In a constant-pressure calorimeter, 47.9 g of X is dissolved in 219 g of water
at 23.00 °C.
X(s)
— Х(аq)
The temperature of the resulting solution rises to 24.30 °C. Assume the solution has the same specific heat as water, 4.184
J/(g.°C), and that there is negligible heat loss to the surroundings.
How much heat was absorbed by the solution?
kJ
What is the enthalpy of the reaction?
ΔΗη
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning