1. When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of solution in a constant-pressure calorimeter, the following reaction occurs: Mg(s) + 2 HCI(aq) → MgCl, (aq) + H2 (8) If the temperature of the solution increases from 23.0 °C to 34.1 °C as a result of this reaction, calculate AH in kJ/mol of Mg. Assume that the solution has a specific heat of 4.18 J/g-°C.
1. When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of solution in a constant-pressure calorimeter, the following reaction occurs: Mg(s) + 2 HCI(aq) → MgCl, (aq) + H2 (8) If the temperature of the solution increases from 23.0 °C to 34.1 °C as a result of this reaction, calculate AH in kJ/mol of Mg. Assume that the solution has a specific heat of 4.18 J/g-°C.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 59E: In a coffee-cup calorimeter, 50.0 mL of 0.100 M AgNO3 and 50.0 mL of 0.100 M HCl are mixed to yield...
Related questions
Question
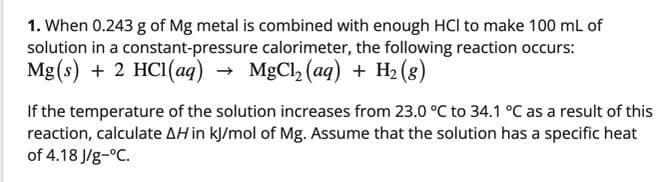
Transcribed Image Text:1. When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of
solution in a constant-pressure calorimeter, the following reaction occurs:
Mg(s) + 2 HCI(aq) → MgCl, (aq) + H2 (8)
If the temperature of the solution increases from 23.0 °C to 34.1 °C as a result of this
reaction, calculate AH in kJ/mol of Mg. Assume that the solution has a specific heat
of 4.18 J/g-°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning