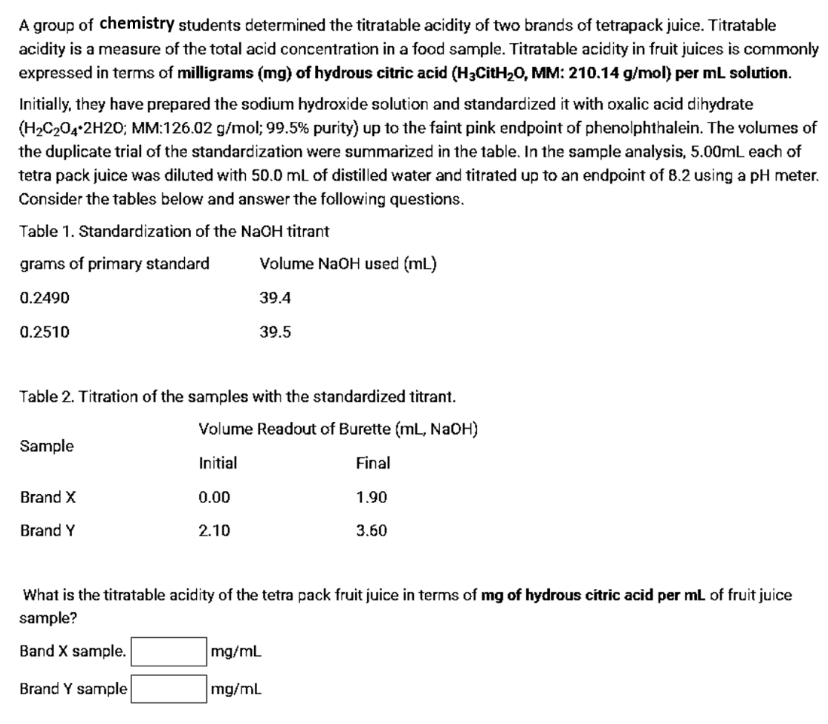
A group of chemistry students determined the titratable acidity of two brands of tetrapack juice. Titratable acidity is a measure of the total acid concentration in a food sample. Titratable acidity in fruit juices is commonly expressed in terms of milligrams (mg) of hydrous citric acid (H3CitH20, MM: 210.14 g/mol) per mL solution. Initially, they have prepared the sodium hydroxide solution and standardized it with oxalic acid dihydrate (H2C204•2H20; MM:126.02 g/mol; 99.5% purity) up to the faint pink endpoint of phenolphthalein. The volumes of the duplicate trial of the standardization were summarized in the table. In the sample analysis, 5.00mL each of tetra pack juice was diluted with 50.0 mL of distilled water and titrated up to an endpoint of 8.2 using a pH meter Consider the tables below and answer the following questions. Table 1. Standardization of the NaOH titrant grams of primary standard Volume NaOH used (mL) 0.2490 39.4 0.2510 39.5 Table 2. Titration of the samples with the standardized titrant. Volume Readout of Burette (mL, NaOH) Sample Initial Final Brand X 0.00 1.90 Brand Y 2.10 3.60 What is the titratable acidity of the tetra pack fruit juice in terms of mg of hydrous citric acid per ml of fruit juice sample? Band X sample. mg/ml Brand Y sample mg/mL
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
Hi! I need help. Answers whould me in mg/ml. Thank u!
Step by step
Solved in 8 steps with 9 images









