You were asked to determine the acid content of vinegar and soda samples. You performed a simple acid-base titration using a solution of NaOH as the titrant with phenolphthalein as the indicator. To determine the concentration of the N2OH solution, you first performed standardization using KHP (FW: 204.22 g/mol, 99.94% purity). For analyzing the samples, he separately measured a volume of vinegar and soda then transferred them to a flask. Complete the table using the provided data. E ISTANDARDIZATION OF NaOH SOLUTION TRIAL 1 0.1012 TRIAL 2 0.1004 TRIAL 3 Weight of KHP, g Initial volume of NaOH, mL Final volume of NaOH, mL 0.09987 5.00 0.00 4.95 10.01 14.93 9.99 0.100 M 0.0985 0.099 M Molarity of NaOH With the data in number one please solve for 0.099 M Average Molarity of NaOH number 2: * ANALYSIS OF CARBONIC ACID IN A SODA SAMPLE ANALYSIS OF CARBONIC TRIAL 1 TRIAL 2 TRIAL 3 ACID IN A SODA SAMPLE Volume of soda, mL Initial volume of NaOH, mL Final volume of NaOH, mL 20.00 18.00 20.00 20.20 24.69 29.48 33.87 24.48 28.50 Molarity of carbonic acid Average molarity of carbonic acid
You were asked to determine the acid content of vinegar and soda samples. You performed a simple acid-base titration using a solution of NaOH as the titrant with phenolphthalein as the indicator. To determine the concentration of the N2OH solution, you first performed standardization using KHP (FW: 204.22 g/mol, 99.94% purity). For analyzing the samples, he separately measured a volume of vinegar and soda then transferred them to a flask. Complete the table using the provided data. E ISTANDARDIZATION OF NaOH SOLUTION TRIAL 1 0.1012 TRIAL 2 0.1004 TRIAL 3 Weight of KHP, g Initial volume of NaOH, mL Final volume of NaOH, mL 0.09987 5.00 0.00 4.95 10.01 14.93 9.99 0.100 M 0.0985 0.099 M Molarity of NaOH With the data in number one please solve for 0.099 M Average Molarity of NaOH number 2: * ANALYSIS OF CARBONIC ACID IN A SODA SAMPLE ANALYSIS OF CARBONIC TRIAL 1 TRIAL 2 TRIAL 3 ACID IN A SODA SAMPLE Volume of soda, mL Initial volume of NaOH, mL Final volume of NaOH, mL 20.00 18.00 20.00 20.20 24.69 29.48 33.87 24.48 28.50 Molarity of carbonic acid Average molarity of carbonic acid
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.130QP: Arsenic acid, H3AsO4, is a poisonous acid that has been used in the treatment of wood to prevent...
Related questions
Question
Please only solve for number two by using the data in number one.
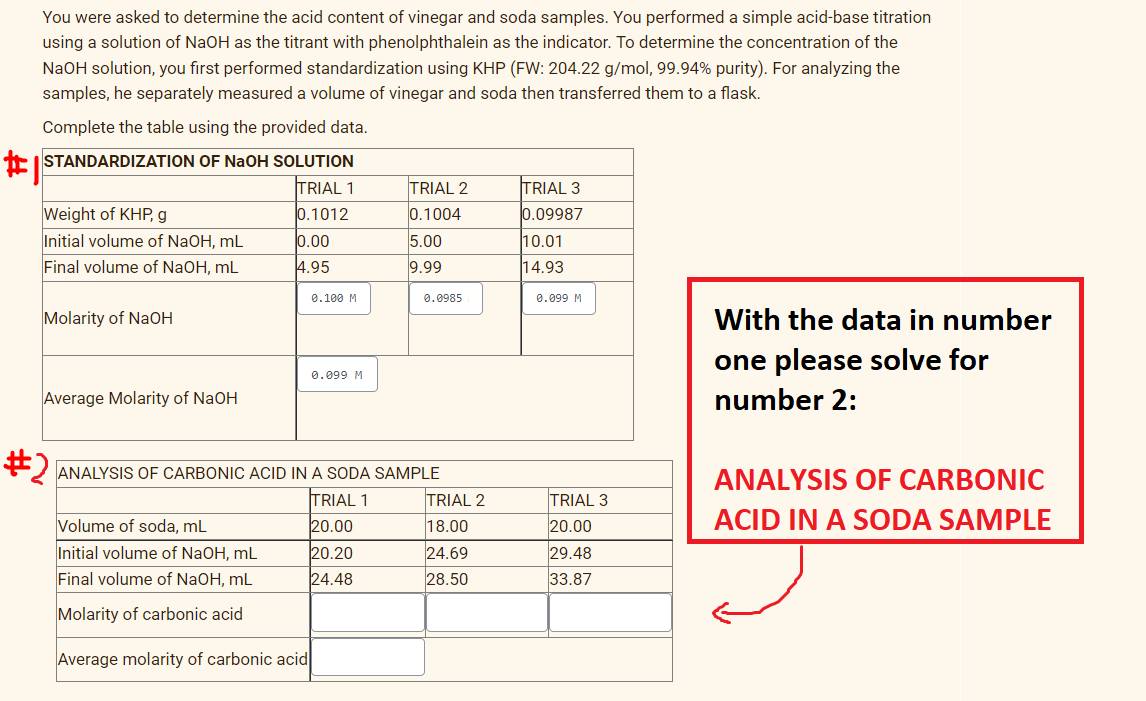
Transcribed Image Text:You were asked to determine the acid content of vinegar and soda samples. You performed a simple acid-base titration
using a solution of NaOH as the titrant with phenolphthalein as the indicator. To determine the concentration of the
NaOH solution, you first performed standardization using KHP (FW: 204.22 g/mol, 99.94% purity). For analyzing the
samples, he separately measured a volume of vinegar and soda then transferred them to a flask.
Complete the table using the provided data.
E ISTANDARDIZATION OF NAOH SOLUTION
TRIAL 1
TRIAL 2
0.1004
TRIAL 3
0.09987
0.1012
Weight of KHP, g
Initial volume of NaOH, mL
Final volume of NaOH, mL
0.00
5.00
10.01
4.95
9.99
14.93
0.100 M
0.0985
0.099 M
Molarity of NaOH
With the data in number
one please solve for
0.099 M
Average Molarity of NaOH
number 2:
ANALYSIS OF CARBONIC ACID IN A SODA SAMPLE
ANALYSIS OF CARBONIC
TRIAL 1
TRIAL 2
TRIAL 3
ACID IN A SODA SAMPLE
Volume of soda, mL
Initial volume of NaOH, mL
Final volume of NaOH, mL
20.00
18.00
20.00
20.20
24.69
29.48
24.48
28.50
33.87
Molarity of carbonic acid
Average molarity of carbonic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning