A group of students is asked to predict which compounds could be added to increase the concentration of dichromate ions, Cr₂O2 (aq), in the chromate/dichromate equilibrium equation represented below. 2 CrO2 (aq) + 2 H(aq) = Cr₂O72-(aq) + H₂O(1) yellow orange Compound 1 K₂CrO4(s) 2 HCl(aq) 3 NaCl(s) 4 H₂O(1) Numerical Response Shift 5 products 6 reactants The colour would become more (Record in the first column) This would cause the equilibrium to shift toward the 12. Using the numbers given above, complete the prediction. To increase the concentration of Cr₂O72-(aq) in the equilibrium system, the compounds that could be added are numbered or (Record in the fourth column) Colour 7 yellow 8 orange (Record in the second column) (Record in the third column) (Record your answer in the numerical-response section on the answer sheet.)
A group of students is asked to predict which compounds could be added to increase the concentration of dichromate ions, Cr₂O2 (aq), in the chromate/dichromate equilibrium equation represented below. 2 CrO2 (aq) + 2 H(aq) = Cr₂O72-(aq) + H₂O(1) yellow orange Compound 1 K₂CrO4(s) 2 HCl(aq) 3 NaCl(s) 4 H₂O(1) Numerical Response Shift 5 products 6 reactants The colour would become more (Record in the first column) This would cause the equilibrium to shift toward the 12. Using the numbers given above, complete the prediction. To increase the concentration of Cr₂O72-(aq) in the equilibrium system, the compounds that could be added are numbered or (Record in the fourth column) Colour 7 yellow 8 orange (Record in the second column) (Record in the third column) (Record your answer in the numerical-response section on the answer sheet.)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 77QRT: Predict whether the equilibrium for the photosynthesis reaction described by the equation
6 CO2(g) +...
Related questions
Question
I need these two
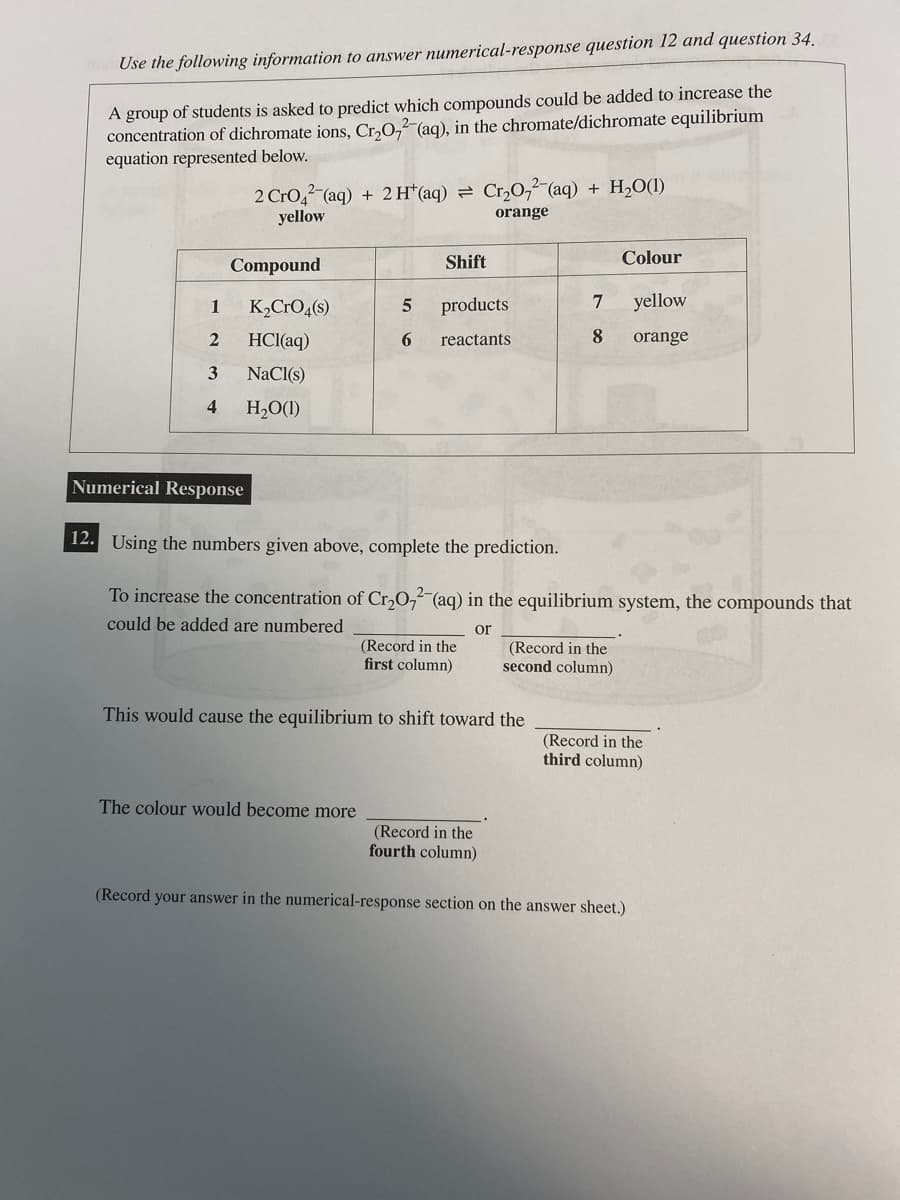
Transcribed Image Text:Use the following information to answer numerical-response question 12 and question 34.
A group of students is asked to predict which compounds could be added to increase the
concentration of dichromate ions, Cr₂O72-(aq), in the chromate/dichromate equilibrium
equation represented below.
1
2
3
4
2 CrO2 (aq) + 2 H+ (aq) = Cr₂O₂(aq) + H₂O(1)
yellow
orange
Compound
Numerical Response
K₂CRO4(s)
HCl(aq)
NaCl(s)
H₂O(1)
5
6
Shift
The colour would become more
products
reactants
(Record in the
first column)
This would cause the equilibrium to shift toward the
7
8
12. Using the numbers given above, complete the prediction.
To increase the concentration of Cr₂O72-(aq) in the equilibrium system, the compounds that
could be added are numbered
or
(Record in the
fourth column)
Colour
(Record in the
second column)
yellow
orange
(Record in the
third column)
(Record your answer in the numerical-response section on the answer sheet.)

Transcribed Image Text:Use the following information to answer numerical-response question 13.
Two Brønsted-Lowry Acid-Base Equilibrium Equations
2-
HC H₂0₂²¯(aq) + H₂O(1) ⇒ C₂H50,³¯(aq) + H₂O¹(aq)
1
2
3
4
HOOCCOO (aq) + H₂O(1) HOOCCOOH(aq) + OH(aq)
5
7
8
Numerical Response
13. In the equilibrium equations above, the species acting as Brønsted-Lowry bases are
numbered
and
(Record all four digits of your answer in any order in the numerical-response section on the answer sheet.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning