A hot air balloon's gondola is suspended below a cloth envelope containing 2.775 x 10° liters of hot air. How many milliliters of hot air is this?
A hot air balloon's gondola is suspended below a cloth envelope containing 2.775 x 10° liters of hot air. How many milliliters of hot air is this?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 94QAP: You want to make a rectangular box that weighs 2.0 pounds and floats on water. The width and height...
Related questions
Question
Transcribed Image Text:h1
10 Chem101
co
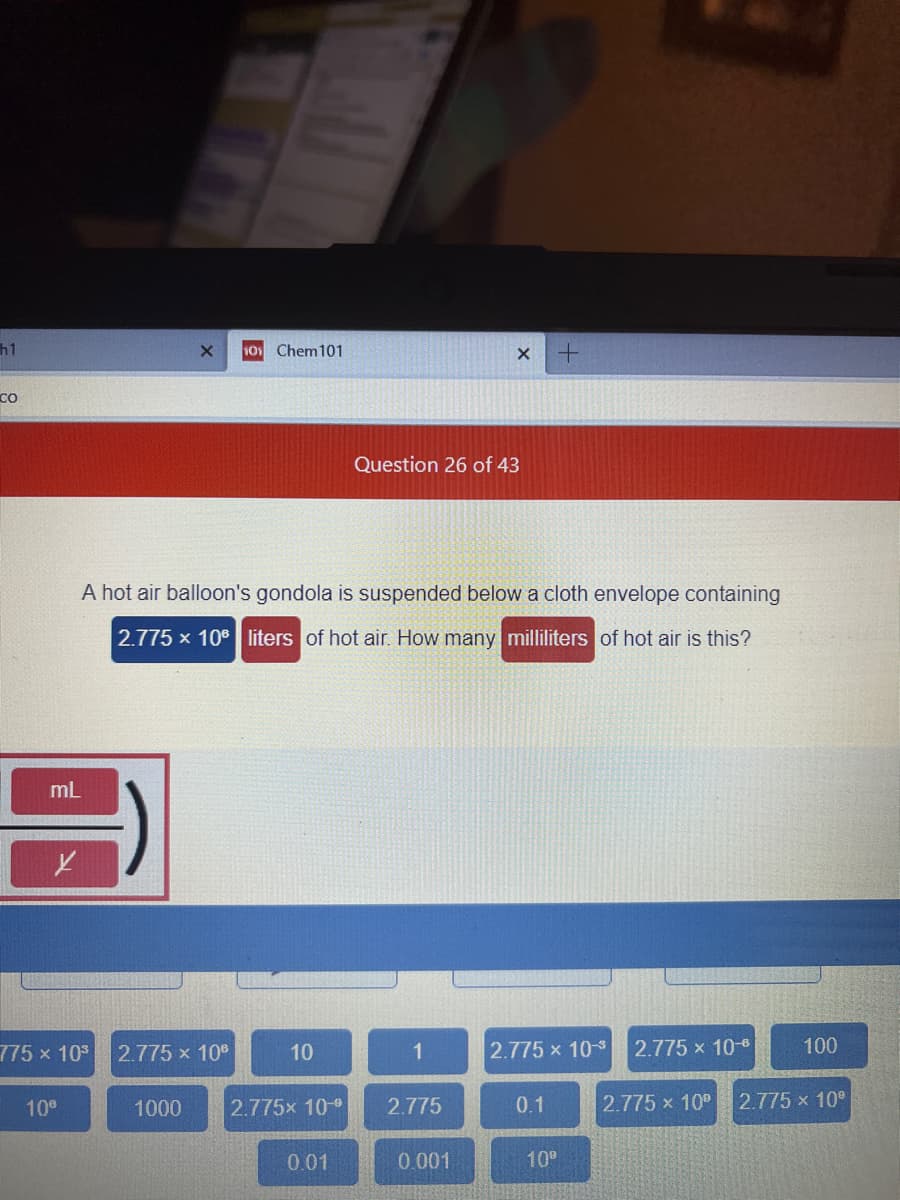
Question 26 of 43
A hot air balloon's gondola is suspended below a cloth envelope containing
2.775 x 10 liters of hot air. How many milliliters of hot air is this?
mL
775 x 10
2.775 x 10°
10
1
2.775 x 103
2.775 x 10-8
100
10
1000
2.775x 10-9
2.775
0.1
2.775 x 10°
2.775 x 10°
0.01
0.001
10
Expert Solution
Step 1
Given,
Volume of the hot air = 2.775 x 106 L
Calculate the volume of the hot air in mL = ?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning