A hot lump of 27.0 g of iron at an initial temperature of 81.7 °C is placed in 50.0 mL H,0 initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is 0.449 J/(g.°C)? Assume no heat is lost to surroundings. Trinal = %3D °C I TOOLS x10 23 9:25 PM N 3/8/2022 DELL End Delete PrtScr Insert Home F11 F12 F10 44 F9 F5 F6 F7 F8 F3 F4 Backs & 23 %24 8. 4
A hot lump of 27.0 g of iron at an initial temperature of 81.7 °C is placed in 50.0 mL H,0 initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is 0.449 J/(g.°C)? Assume no heat is lost to surroundings. Trinal = %3D °C I TOOLS x10 23 9:25 PM N 3/8/2022 DELL End Delete PrtScr Insert Home F11 F12 F10 44 F9 F5 F6 F7 F8 F3 F4 Backs & 23 %24 8. 4
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 64E: A 110.-g sample of copper (specific heat capacity = 0.20 J/C g) is heated to 82.4C and then placed...
Related questions
Question
100%
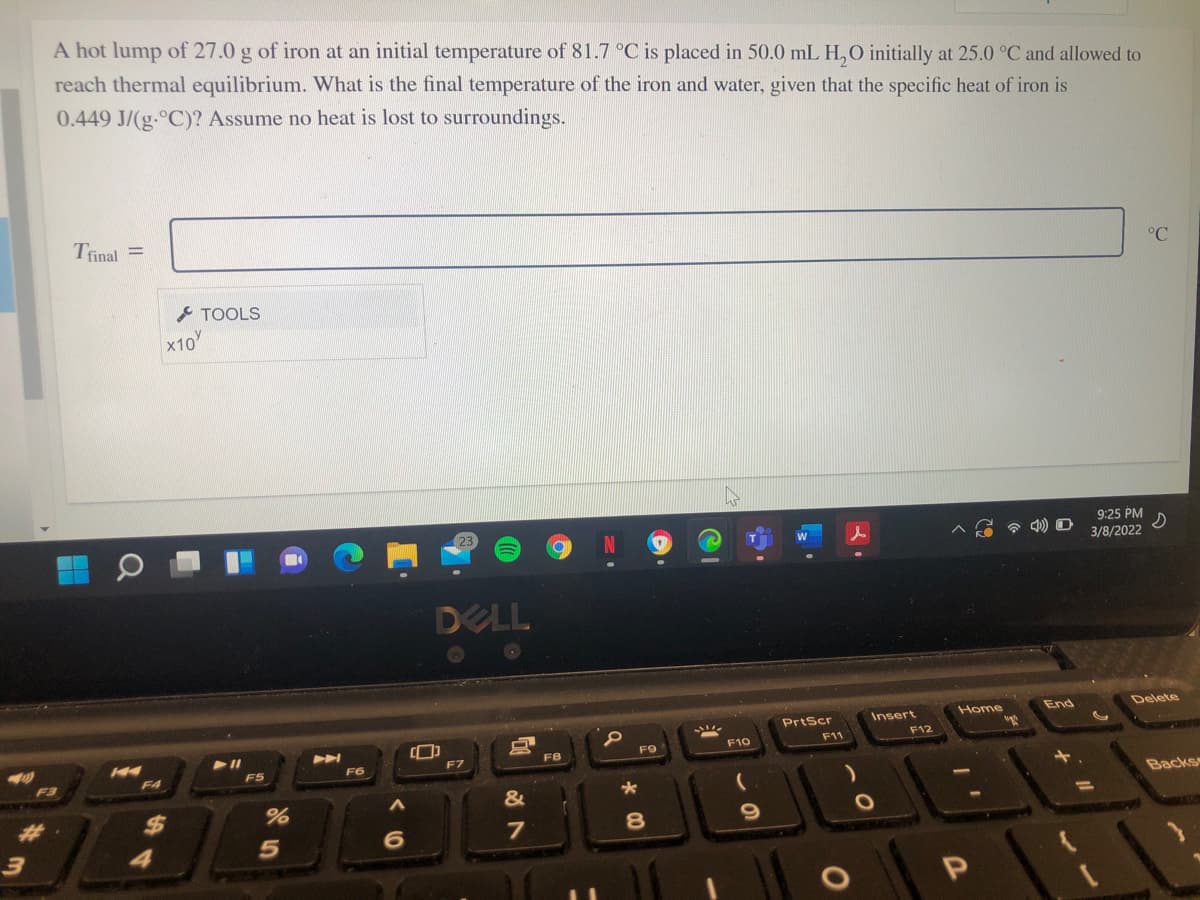
Transcribed Image Text:A hot lump of 27.0 g of iron at an initial temperature of 81.7 °C is placed in 50.0 mL H,0 initially at 25.0 °C and allowed to
reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is
0.449 J/(g.°C)? Assume no heat is lost to surroundings.
Trinal =
°C
I TOOLS
x10
23
9:25 PM
人
* 4) O
3/8/2022
DELL
End
Delete
PrtScr
Insert
Home
F11
F12
F10
K4
F9
F8
F5
F6
F7
F3
F4
Backs
&
23
%24
8.
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning