A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following table. Action Enthalpy separation of solute 18.5 kJ/mol separation of solvent 19.8 kJ/mol formation of solute-solvent interactions -84.7 kJ/mol solute Calculate the enthalpy of solution in kilojoules per mole of solute.
A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following table. Action Enthalpy separation of solute 18.5 kJ/mol separation of solvent 19.8 kJ/mol formation of solute-solvent interactions -84.7 kJ/mol solute Calculate the enthalpy of solution in kilojoules per mole of solute.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.55E
Related questions
Question
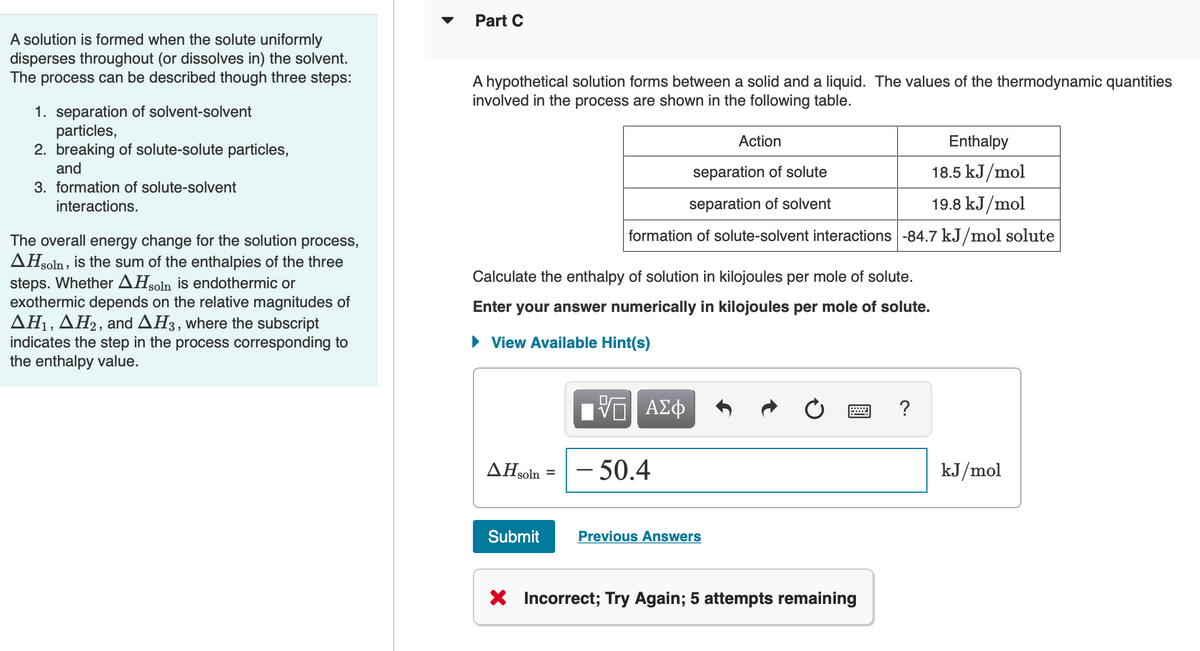
Transcribed Image Text:Part C
A solution is formed when the solute uniformly
disperses throughout (or dissolves in) the solvent.
The process can be described though three steps:
A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities
involved in the process are shown in the following table.
1. separation of solvent-solvent
particles,
2. breaking of solute-solute particles,
and
Action
Enthalpy
separation of solute
18.5 kJ/mol
3. formation of solute-solvent
interactions.
separation of solvent
19.8 kJ/mol
formation of solute-solvent interactions -84.7 kJ/mol solute
The overall energy change for the solution process,
AHsoln , is the sum of the enthalpies of the three
steps. Whether AHsoln is endothermic or
exothermic depends on the relative magnitudes of
AH1, AH2, and AH3, where the subscript
indicates the step in the process corresponding to
the enthalpy value.
Calculate the enthalpy of solution in kilojoules per mole of solute.
Enter your answer numerically in kilojoules per mole of solute.
• View Available Hint(s)
V ΑΣφ
?
AHsoln
– 50.4
kJ/mol
%3D
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning