(a) i (b) II (e) I and II 0) (c) II (f) none are correct aorgridi obt 15. Provide the missing reagents for the following reaction. OH Et (a) H2SO4, EtOH (d 1. LiAIHi 2.H30+ workup (b) Na O Et, EtOH me to (e) 1. EtMgBr; 2. H30+ workup (c) PCC (f) None of them HO,MO H la HOO
(a) i (b) II (e) I and II 0) (c) II (f) none are correct aorgridi obt 15. Provide the missing reagents for the following reaction. OH Et (a) H2SO4, EtOH (d 1. LiAIHi 2.H30+ workup (b) Na O Et, EtOH me to (e) 1. EtMgBr; 2. H30+ workup (c) PCC (f) None of them HO,MO H la HOO
Chapter30: Orbitals And Organic Chemistry: Pericyclic Reactions
Section30.SE: Something Extra
Problem 41AP: In light of your answer to Problem 30-40, explain why a mixture of products occurs in the following...
Related questions
Question
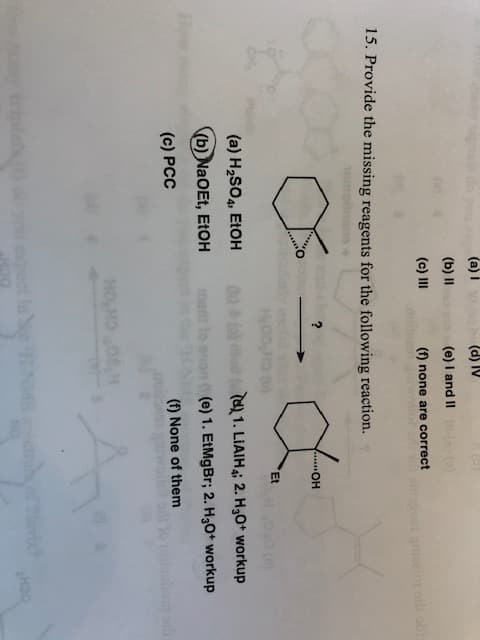
15. provide the missing reagents for the following reaction
Transcribed Image Text:(a) i
(b) II
(e) I and II
0)
(c) II
(f) none are correct
aorgridi obt
15. Provide the missing reagents for the following reaction.
OH
Et
(a) H2SO4, EtOH
(d 1. LiAIHi 2.H30+ workup
(b) Na O Et, EtOH
me to
(e) 1. EtMgBr; 2. H30+ workup
(c) PCC
(f) None of them
HO,MO
H
la
HOO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

