A lactic acid/lactate ion buffer solution contains 0.21 MHC₂H5O3 and 0.71 MC₂H5O₁, respectively. The K, value of lac acid is 1.4 x 10-4. Calculate the pH of this buffer. Express the pH numerically. silable Hint(e)
A lactic acid/lactate ion buffer solution contains 0.21 MHC₂H5O3 and 0.71 MC₂H5O₁, respectively. The K, value of lac acid is 1.4 x 10-4. Calculate the pH of this buffer. Express the pH numerically. silable Hint(e)
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.22QAP
Related questions
Question
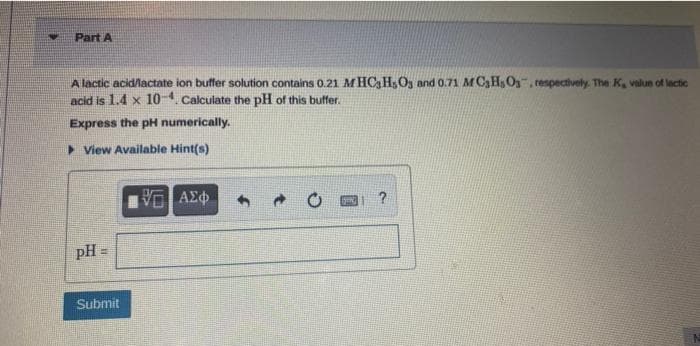
Transcribed Image Text:Part A
A lactic acid/lactate ion buffer solution contains 0.21 MHC HsO3 and 0.71 MC3Hs Os, respectively. The K, value of lactic
acid is 1.4 x 10-¹. Calculate the pH of this buffer.
Express the pH numerically.
▸ View Available Hint(s)
pH =
Submit
[ΕΙ ΑΣΦ
?
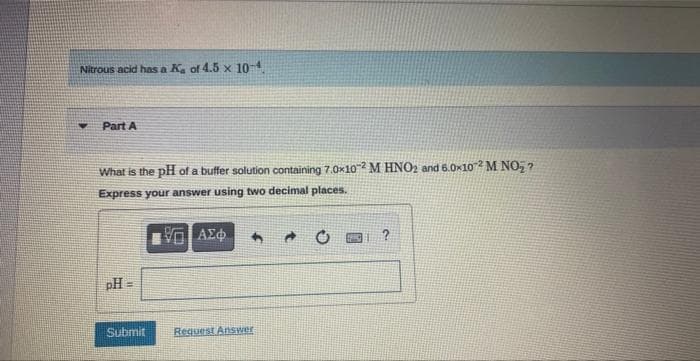
Transcribed Image Text:Nitrous acid has a Ka of 4.5 x 10-4.
▼
Part A
What is the pH of a buffer solution containing 7.0x102 M HNO2 and 6.0x102 M NO₂?
Express your answer using two decimal places.
pH =
Submit
VO ΑΣΦ
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



