A logical consequence of the second law of thermodynamics could be stated, O if there is an increase in the energy of a system, there must be a corresponding decrease in the energy of the rest of the universe. O energy can be transferred or transformed, but it cannot be created or destroyed. O every energy transfer requires activation energy from the environment. O if the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe. O a spontaneous chemical reaction must increase the total entropy of the universe.
A logical consequence of the second law of thermodynamics could be stated, O if there is an increase in the energy of a system, there must be a corresponding decrease in the energy of the rest of the universe. O energy can be transferred or transformed, but it cannot be created or destroyed. O every energy transfer requires activation energy from the environment. O if the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe. O a spontaneous chemical reaction must increase the total entropy of the universe.
Biology Today and Tomorrow without Physiology (MindTap Course List)
5th Edition
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cecie Starr, Christine Evers, Lisa Starr
Chapter4: Energy And Metabolism
Section: Chapter Questions
Problem 1FIO: Figure 4.5 Energy inputs and outputs in chemical reactions. 1 Some reactions convert molecules with...
Related questions
Question
i need help finding the right answer with an explnation please
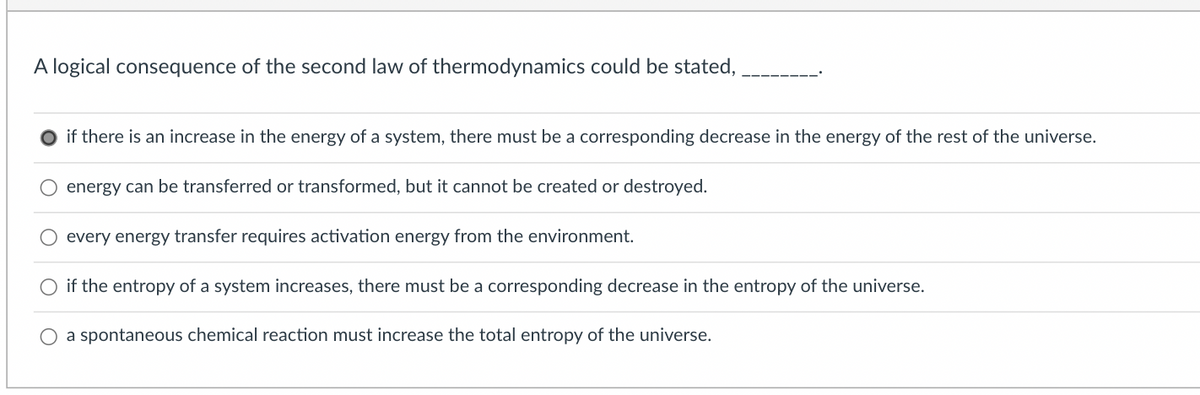
Transcribed Image Text:A logical consequence of the second law of thermodynamics could be stated,
if there is an increase in the energy of a system, there must be a corresponding decrease in the energy of the rest of the universe.
energy can be transferred or transformed, but it cannot be created or destroyed.
every energy transfer requires activation energy from the environment.
O if the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe.
a spontaneous chemical reaction must increase the total entropy of the universe.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning


Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning


Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Principles Of Radiographic Imaging: An Art And A …
Health & Nutrition
ISBN:
9781337711067
Author:
Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:
Cengage Learning