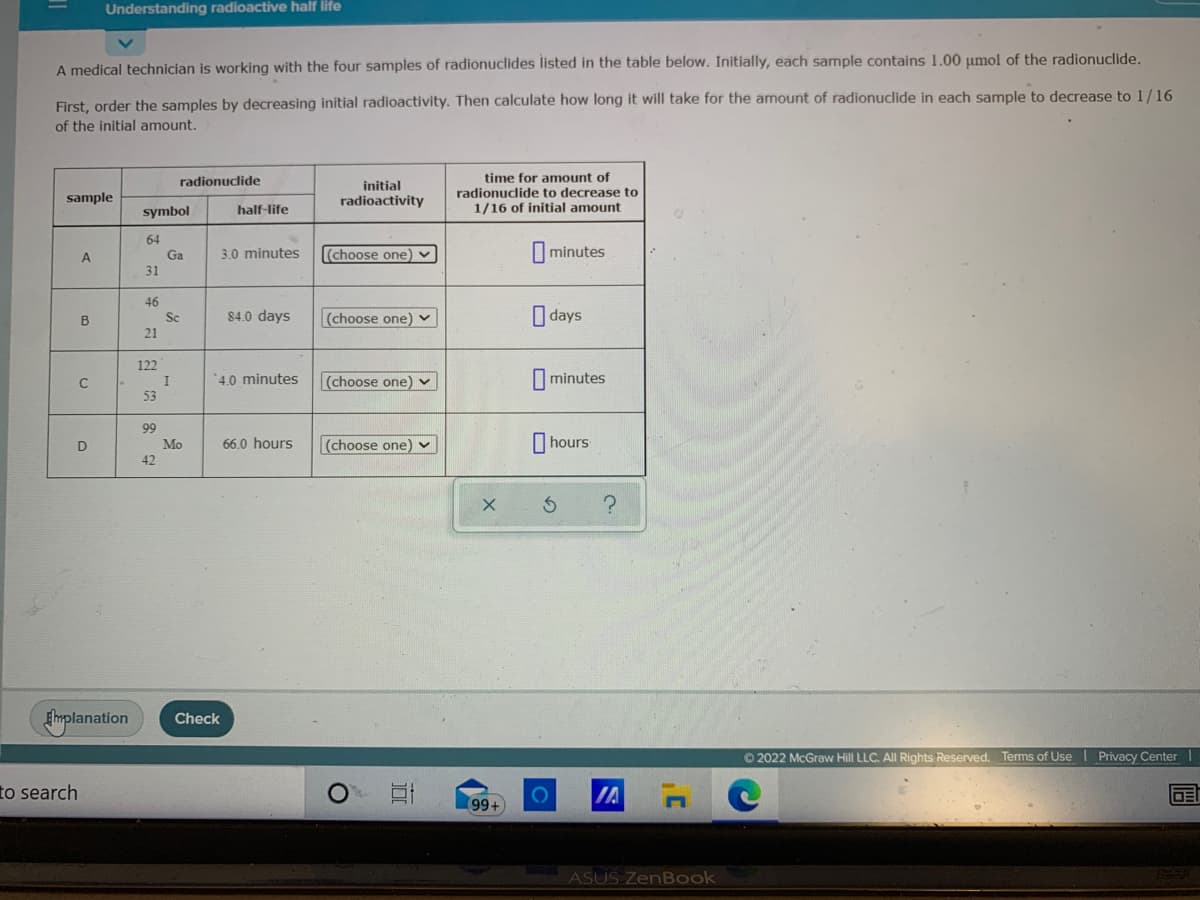
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 1.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. time for amount of radionucdlide to decrease to 1/16 of initial amount radionuclide initial sample radioactivity symbol half-life 64 Ga (choose one) - |minutes A 3.0 minutes 31 46 Sc 21 O days 84.0 days (choose one) v 122 I 53 4.0 minutes (choose one) v I minutes 99 Mo 42 D 66.0 hours (choose one) v I hours
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 1.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. time for amount of radionucdlide to decrease to 1/16 of initial amount radionuclide initial sample radioactivity symbol half-life 64 Ga (choose one) - |minutes A 3.0 minutes 31 46 Sc 21 O days 84.0 days (choose one) v 122 I 53 4.0 minutes (choose one) v I minutes 99 Mo 42 D 66.0 hours (choose one) v I hours
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section19.2: Application Of Radioactivity
Problem 4RQ
Related questions
Question
Transcribed Image Text:Understanding radioactive half lite
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 1.00 µmol of the radionuclide.
First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16
of the initial amount.
time for amount of
radionuclide to decrease to
1/16 of initial amount
radionucdlide
initial
sample
radioactivity
symbol
half-life
64
Ga
A
3.0 minutes
(choose one) v
minutes
31
46
Sc
84.0 days
(choose one) v
O days
B
21
122
'4.0 minutes
|(choose one) v
I minutes
I
53
99
66.0 hours
(choose one) v
I hours
Mo
42
Smplanation
Check
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use l Privacy Center I
to search
IA
99+
ASUS ZenBook
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning