A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 3.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/8 of the initial amount. 20 time for amount of radionuclide initial radionuclide to decrease to 1/8 of initial amount sample radioactivity symbol half-life 59 days V (choose me) 1 (highest Fe 45.0 days 26 2. 178 Ta I minutes 9.0 minutes 4 (lowest) B 73 139 Ce I days 138. days (choose one) 58 60 I years Co 5.0 years (choose one) 27
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 3.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/8 of the initial amount. 20 time for amount of radionuclide initial radionuclide to decrease to 1/8 of initial amount sample radioactivity symbol half-life 59 days V (choose me) 1 (highest Fe 45.0 days 26 2. 178 Ta I minutes 9.0 minutes 4 (lowest) B 73 139 Ce I days 138. days (choose one) 58 60 I years Co 5.0 years (choose one) 27
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter20: Nuclear Chemistry
Section: Chapter Questions
Problem 20.27QP
Related questions
Question
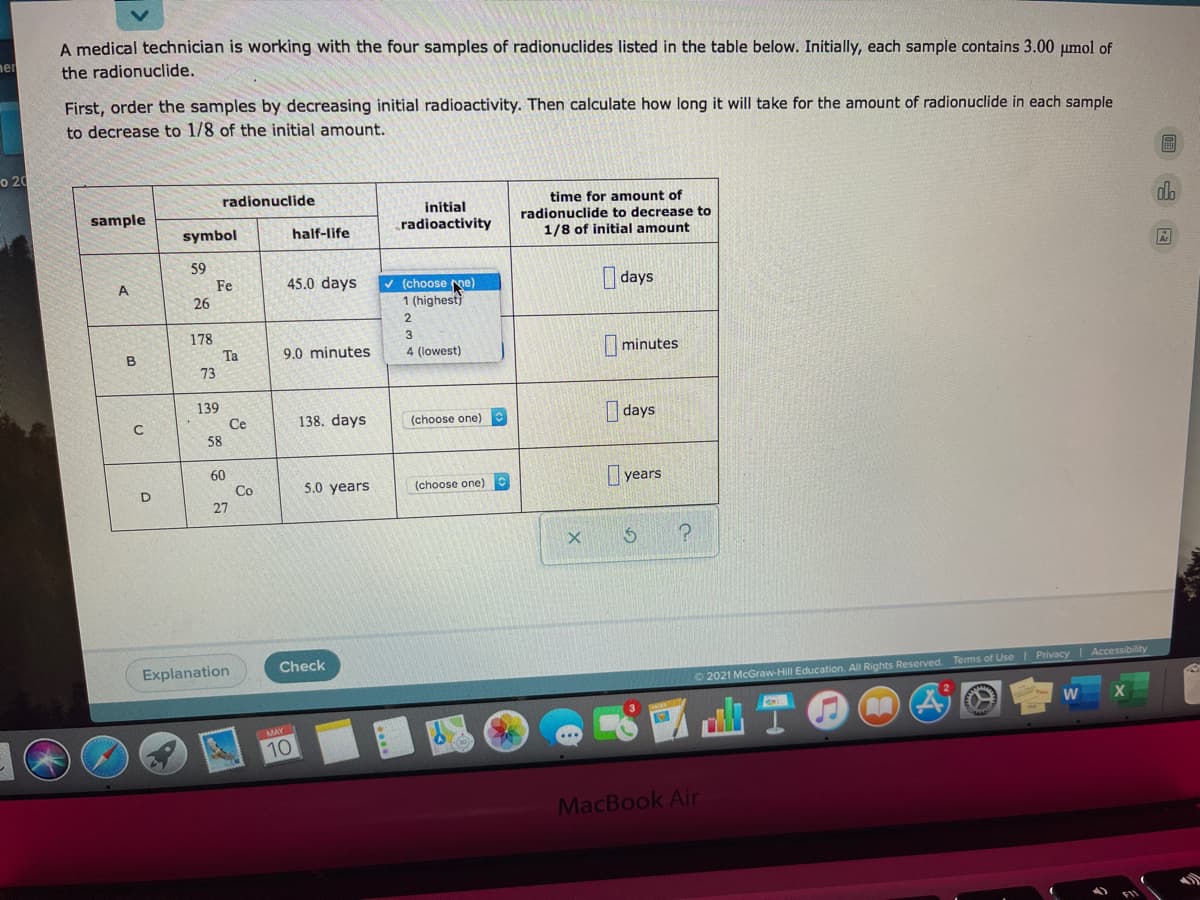
Transcribed Image Text:A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 3.00 umol of
ner
the radionuclide.
First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample
to decrease to 1/8 of the initial amount.
圖
o 20
radionuclide
time for amount of
initial
radioactivity
sample
radionuclide to decrease to
1/8 of initial amount
symbol
half-life
59
45.0 days
days
V (choose Noe)
1 (highest)
A
Fe
26
178
3
Ta
9.0 minutes
4 (lowest)
minutes
B
73
139
138. days
(choose one)
I days
Ce
58
60
Co
27
|years
5.0 years
(choose one)
D
Check
Explanation
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of UseI Privacy Accessibility
MAY
10
MacBook Air
(4)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning