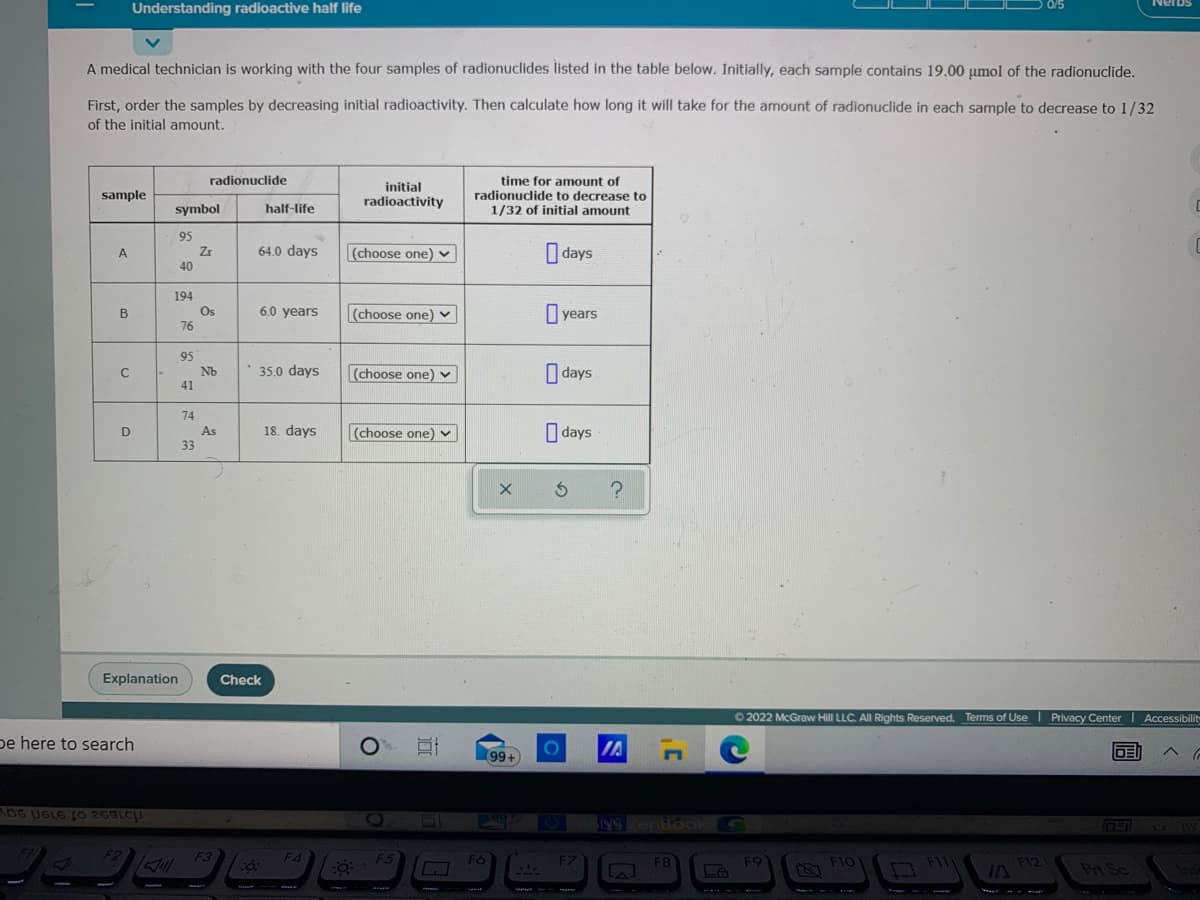
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 19.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. time for amount of radionudide to decrease to 1/32 of initial amount radionuclide initial radioactivity sample symbol half-life 95 Zr O days A 64.0 days (choose one) v 40 194 Os 76 6.0 years (choose one) v years B 95 35.0 days O days Nb (choose one) v 41 74 18. days (choose one) v O days D As 33
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 19.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. time for amount of radionudide to decrease to 1/32 of initial amount radionuclide initial radioactivity sample symbol half-life 95 Zr O days A 64.0 days (choose one) v 40 194 Os 76 6.0 years (choose one) v years B 95 35.0 days O days Nb (choose one) v 41 74 18. days (choose one) v O days D As 33
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter20: Nuclear Chemistry
Section: Chapter Questions
Problem 20.27QP
Related questions
Question
Transcribed Image Text:Nerbs
IDS
Understanding radioactive half life
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 19.00 umol of the radionuclide.
First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32
of the initial amount.
radionuclide
time for amount of
radionuclide to decrease to
1/32 of initial amount
initial
sample
radioactivity
symbol
half-life
95
Zr
40
64.0 days
(choose one) v
I days
A
194
Os
76
6.0 years
(choose one) v
O years
B
95
Nb
35.0 days
(choose one) v
I days
41
74
18. days
O days
D
As
(choose one) v
33
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center I Accessibilit
pe here to search
画 へG
99+
[O
CHO
NS ZenBook G
F3
F4
F5
F6
F10
F7
F8
F9
F12
Prt Sc
LAJ
. ..nt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning