A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 17.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B C D symbol 52 25 15 8 212 82 91 39 radionuclide Mn O Pb Y half-life 6.0 days 122. seconds 11. hours 59.0 days initial radioactivity (choose one) (choose one) (choose one) O (choose one) time for amount of radionuclide to decrease to 1/32 of initial amount X days seconds hours days Ar
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 17.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B C D symbol 52 25 15 8 212 82 91 39 radionuclide Mn O Pb Y half-life 6.0 days 122. seconds 11. hours 59.0 days initial radioactivity (choose one) (choose one) (choose one) O (choose one) time for amount of radionuclide to decrease to 1/32 of initial amount X days seconds hours days Ar
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section: Chapter Questions
Problem 67IL
Related questions
Question
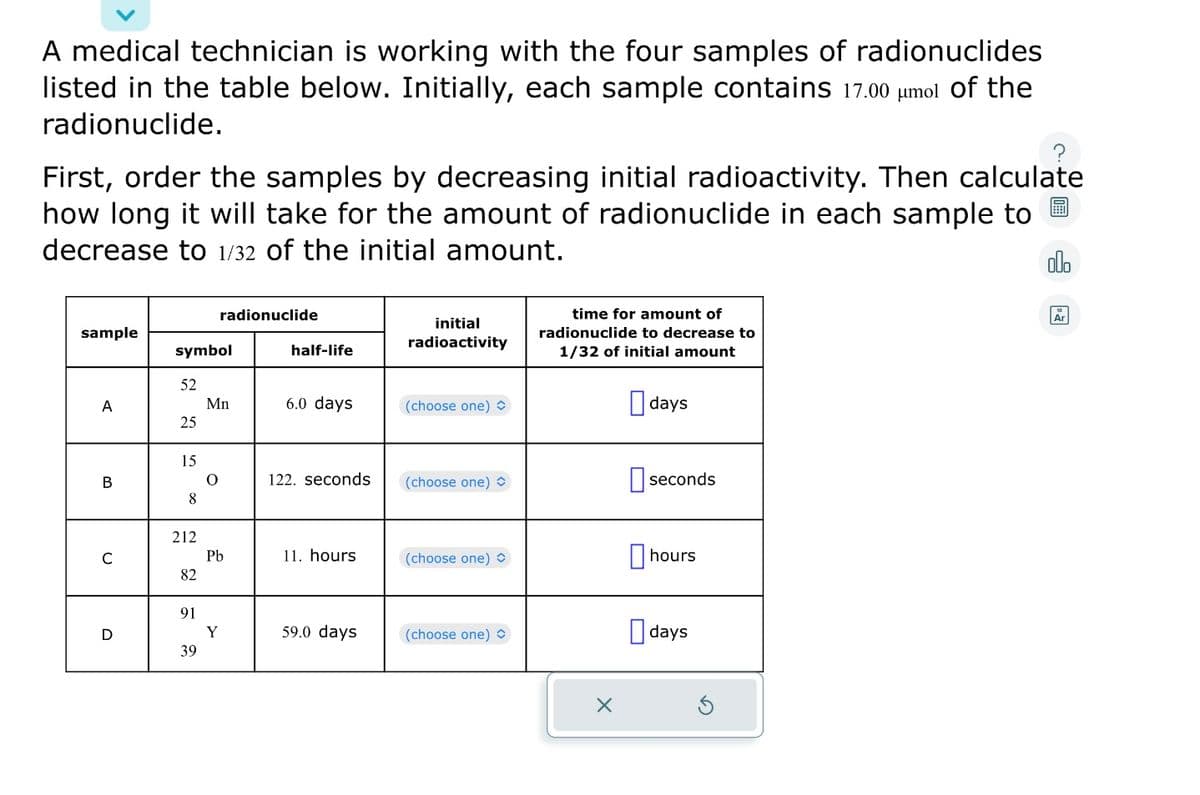
Transcribed Image Text:A medical technician is working with the four samples of radionuclides
listed in the table below. Initially, each sample contains 17.00 μmol of the
radionuclide.
First, order the samples by decreasing initial radioactivity. Then calculate
how long it will take for the amount of radionuclide in each sample to
decrease to 1/32 of the initial amount.
olo
sample
A
B
C
D
symbol
52
25
15
8
212
82
91
39
radionuclide
Mn
O
Pb
Y
half-life
6.0 days
122. seconds
11. hours
59.0 days
initial
radioactivity
(choose one)
(choose one)
(choose one)
(choose one) >
time for amount of
radionuclide to decrease to
1/32 of initial amount
X
days
seconds
hours
days
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
