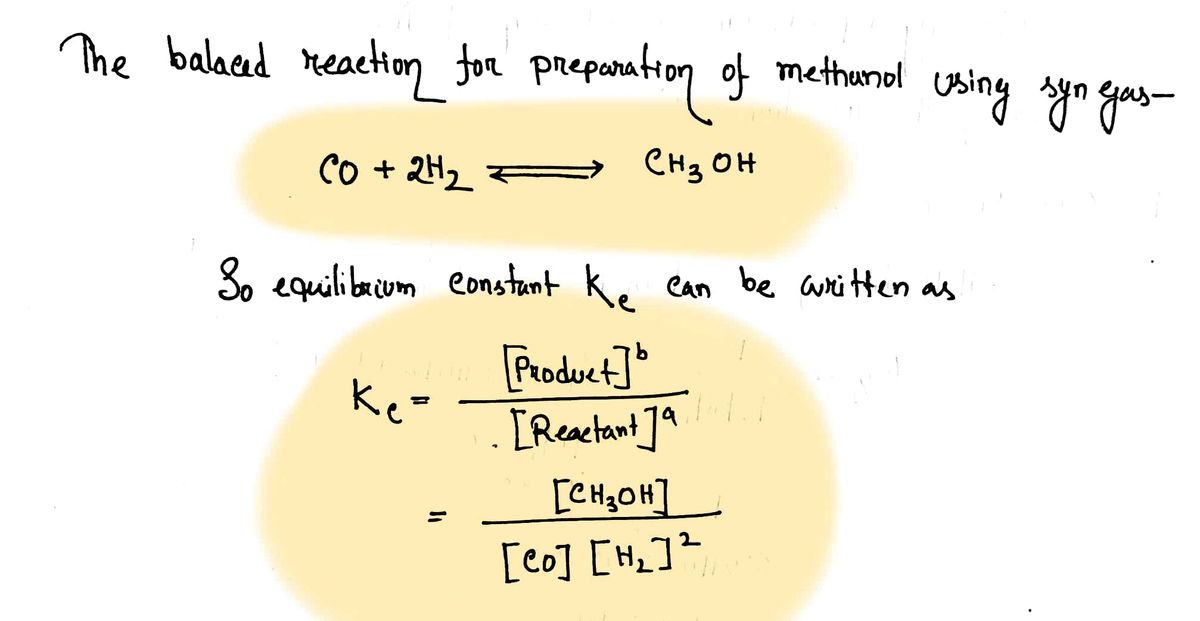
A mixture of gaseous CO and H2, called synthesis gas, is used commercially to prepare methanol (CH3OH), a compound considered an alternative fuel to gasoline. Under equilibrium conditions at 565.3 K, [H2] = 0.07911 mol/L, [CO] = 0.03125 mol/L, and [CH3OH] = 0.0401 mol/L. What is the value of K. for this reaction at 565.3 K?
A mixture of gaseous CO and H2, called synthesis gas, is used commercially to prepare methanol (CH3OH), a compound considered an alternative fuel to gasoline. Under equilibrium conditions at 565.3 K, [H2] = 0.07911 mol/L, [CO] = 0.03125 mol/L, and [CH3OH] = 0.0401 mol/L. What is the value of K. for this reaction at 565.3 K?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 117AP
Related questions
Question
![A mixture of gaseous CO and H2, called synthesis gas, is used commercially to prepare methanol (CH3OH), a compound considered an
alternative fuel to gasoline. Under equilibrium conditions at 565.3 K,[H2] = 0.07911 mol/L, [CO] = 0.03125 mol/L, and [CH3OH] =
0.0401 mol/L. What is the value of K. for this reaction at 565.3 K?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3595772e-86c0-4c31-a854-eeb2464bab68%2Ff5cf5b5b-45b1-4eb9-a5dd-0fa0d4c6d7f9%2F2zcdevd_processed.png&w=3840&q=75)
Transcribed Image Text:A mixture of gaseous CO and H2, called synthesis gas, is used commercially to prepare methanol (CH3OH), a compound considered an
alternative fuel to gasoline. Under equilibrium conditions at 565.3 K,[H2] = 0.07911 mol/L, [CO] = 0.03125 mol/L, and [CH3OH] =
0.0401 mol/L. What is the value of K. for this reaction at 565.3 K?
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning