A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The 1 atm pressure piston cylinder is also submerged in a large insulated water bath. (See sketch at right.) cylinder The temperature of the water bath is monitored, and it is determined from this data that 253. kJ of heat flows into the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the system does 383. kJ of work on the piston during the reaction. water bath gases O exothermic Is the reaction exothermic or endothermic? O endothermic O up ? Does the temperature of the water bath go up or down? O down O neither O in Does the piston move in or out? O out O neither O absorb Does the reaction absorb or release energy? O release O neither How much energy does the reaction absorb or release? Be sure your answer has the correct number of significant digits.
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The 1 atm pressure piston cylinder is also submerged in a large insulated water bath. (See sketch at right.) cylinder The temperature of the water bath is monitored, and it is determined from this data that 253. kJ of heat flows into the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the system does 383. kJ of work on the piston during the reaction. water bath gases O exothermic Is the reaction exothermic or endothermic? O endothermic O up ? Does the temperature of the water bath go up or down? O down O neither O in Does the piston move in or out? O out O neither O absorb Does the reaction absorb or release energy? O release O neither How much energy does the reaction absorb or release? Be sure your answer has the correct number of significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.97PAE: 97 Homes in rural areas where natural gas service is not available often rely on propane to fuel...
Related questions
Question
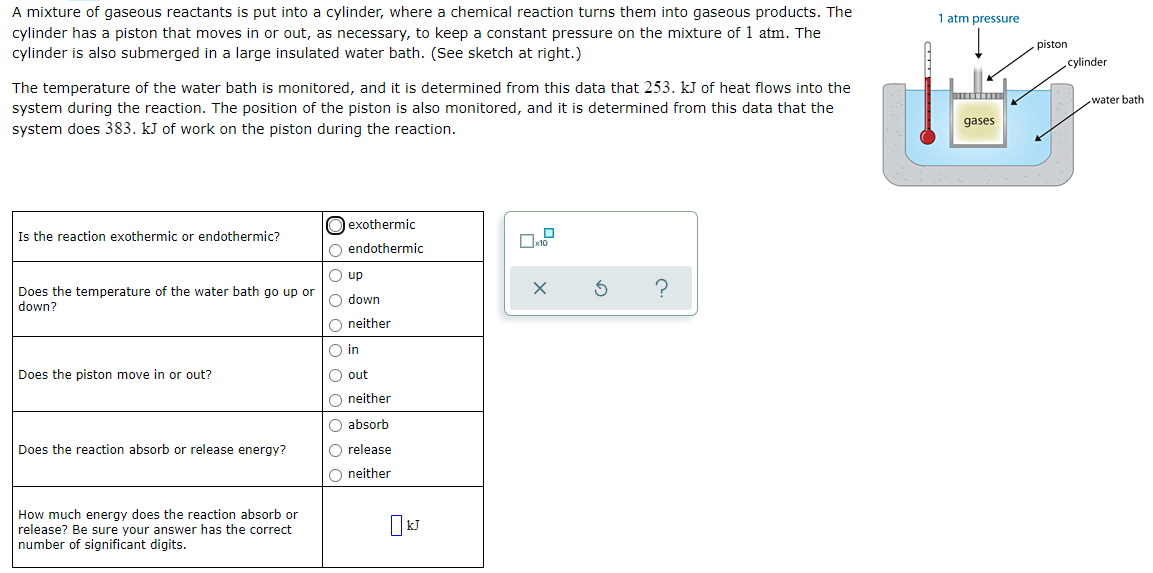
Transcribed Image Text:A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The
cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The
1 atm pressure
piston
cylinder is also submerged in a large insulated water bath. (See sketch at right.)
cylinder
The temperature of the water bath is monitored, and it is determined from this data that 253. kJ of heat flows into the
system during the reaction. The position of the piston is also monitored, and it is determined from this data that the
system does 383. kJ of work on the piston during the reaction.
water bath
gases
O exothermic
Is the reaction exothermic or endothermic?
O endothermic
O up
?
Does the temperature of the water bath go up or
down?
O down
O neither
O in
Does the piston move in or out?
O out
O neither
O absorb
Does the reaction absorb or release energy?
O release
O neither
How much energy does the reaction absorb or
release? Be sure your answer has the correct
number of significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning