Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.23QAP
Related questions
Question
Choose the correct answer ans write your solution.
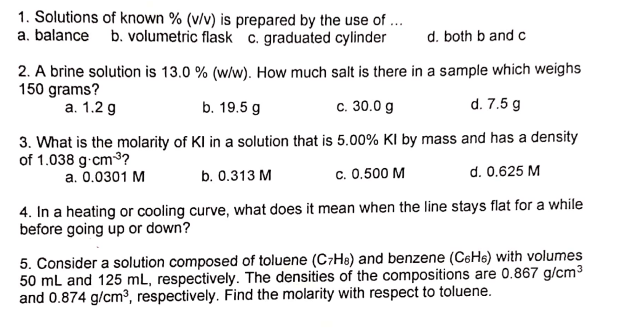
Transcribed Image Text:1. Solutions of known % (v/v) is prepared by the use of ...
a. balance b. volumetric flask c. graduated cylinder
d. both b and c
2. A brine solution is 13.0 % (w/w). How much salt is there in a sample which weighs
150 grams?
а. 1.2 g
b. 19.5 g
c. 30.0 g
d. 7.5 g
3. What is the molarity of KI in a solution that is 5.00% KI by mass and has a density
of 1.038 g.cm3?
b. 0.313 M
c. 0.500 M
d. 0.625 M
a. 0.0301 M
4. In a heating or cooling curve, what does it mean when the line stays flat for a while
before going up or down?
5. Consider a solution composed of toluene (C7He) and benzene (C6H6) with volumes
50 mL and 125 mL, respectively. The densities of the compositions are 0.867 g/cm3
and 0.874 g/cm³, respectively. Find the molarity with respect to toluene.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning