A mixture of three compounds, A, B, and C is to be separated and purified by crystallization Their solubilities in g/100mL ethanol are given below: Solvent A Cold 1.05 5.60 4.20 Hot 20.5 2.3 3.50 a. If a mixture containing 4.0 g of each A, B and C is recrystallized from 100 mL ethanol, which compound would be obtained pure? b. If the filtrate from (a) is evaporated to 50 mL, which of the compound(s) will separate upon cooling? What is its purity?
A mixture of three compounds, A, B, and C is to be separated and purified by crystallization Their solubilities in g/100mL ethanol are given below: Solvent A Cold 1.05 5.60 4.20 Hot 20.5 2.3 3.50 a. If a mixture containing 4.0 g of each A, B and C is recrystallized from 100 mL ethanol, which compound would be obtained pure? b. If the filtrate from (a) is evaporated to 50 mL, which of the compound(s) will separate upon cooling? What is its purity?
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter7: Extraction
Section: Chapter Questions
Problem 1Q
Related questions
Question
Provide solution and explanation for the problem below.
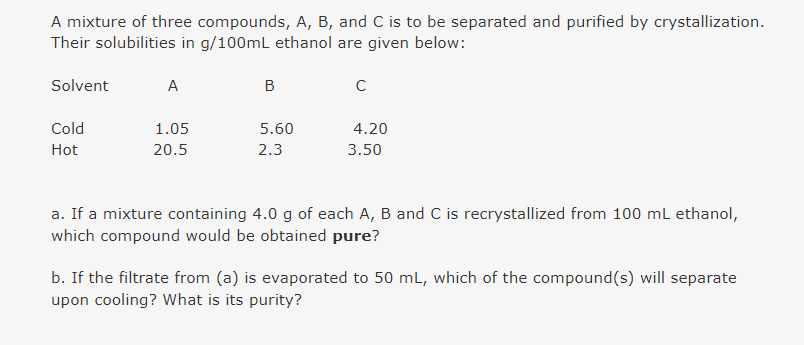
Transcribed Image Text:A mixture of three compounds, A, B, and C is to be separated and purified by crystallization.
Their solubilities in g/100ml ethanol are given below:
Solvent
A
B
Cold
1.05
5.60
4.20
Hot
20.5
2.3
3.50
a. If a mixture containing 4.0 g of each A, B and C is recrystallized from 100 mL ethanol,
which compound would be obtained pure?
b. If the filtrate from (a) is evaporated to 50 mL, which of the compound(s) will separate
upon cooling? What is its purity?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole